IB MYP 4-5 Chemistry -Chromatography and applications- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Chromatography and applications- Study Notes
Key Concepts
- Chromatography and Its Applications
Chromatography and Its Applications
Chromatography and Its Applications
Chromatography is a physical separation technique used to separate and identify different substances present in a mixture, especially when they are soluble in the same solvent and present in small quantities. It is based on the principle that different substances move at different speeds through a medium.
Principle of Chromatography
Chromatography works on the principle of differential adsorption or different solubilities of substances in two phases:
- Stationary phase: The surface or material that stays fixed (e.g., filter paper).
- Mobile phase: The solvent that moves through the stationary phase carrying the components of the mixture.
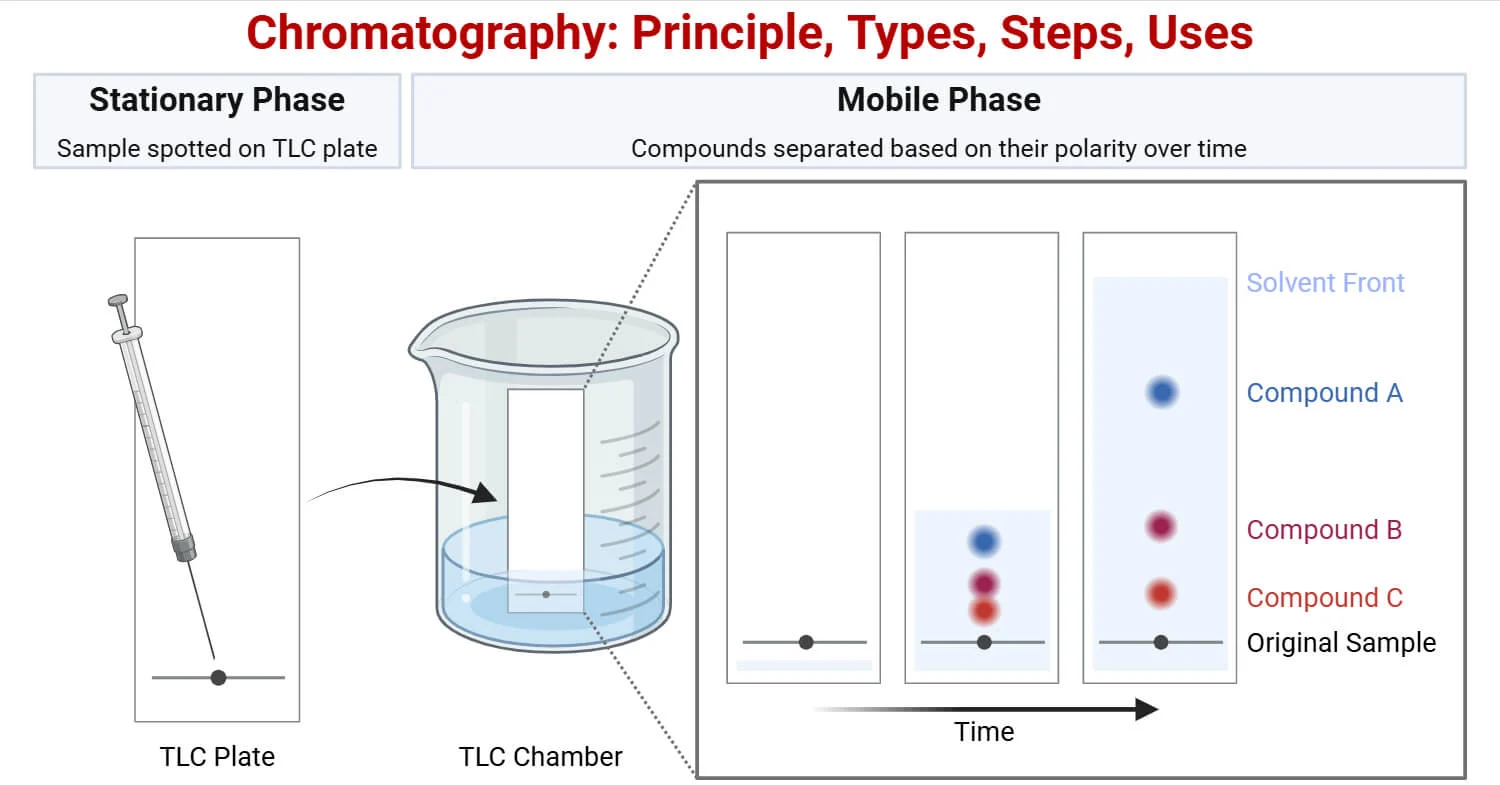
Different substances in the mixture travel at different speeds because of differences in:
- Solubility in the solvent (mobile phase).
- Attraction / adsorption to the stationary phase.
Thus, substances separate into distinct colored spots or bands.
Types of Chromatography (Overview)
| Type | Stationary Phase | Mobile Phase | Common Use |
|---|---|---|---|
| Filter paper | Solvent (e.g., water, alcohol) | Separating dyes and pigments |
Thin Layer Chromatography (TLC) | Glass plate coated with silica gel | Suitable organic solvent | Testing purity and identifying compounds |
| Packed column with solid adsorbent (e.g., silica) | Solvent passing through column | Large-scale separation in labs or industry |
Paper Chromatography (MYP Focus Method)
Steps of Paper Chromatography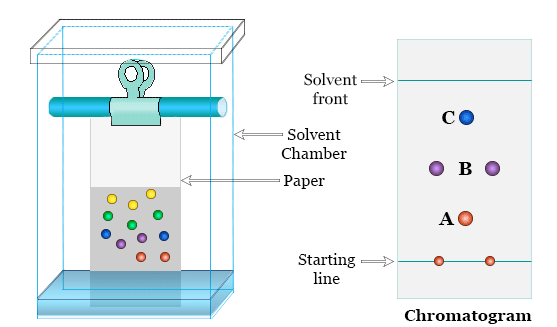
- Draw a pencil line near the bottom of a strip of filter paper (baseline).
- Place small spots of the mixture (e.g., ink or dye) on the line.
- Dip the lower edge of the paper into a suitable solvent — but ensure the spots do not touch the solvent initially.
- The solvent travels up the paper by capillary action, carrying the mixture’s components at different rates.
- When the solvent has moved sufficiently, remove and dry the paper to observe separated spots.
The final paper with separated spots is called a chromatogram.
Observation
- Different components rise to different heights depending on their solubility and interaction with the paper.
- More soluble substances move farther up; less soluble ones stay near the baseline.
Retention Factor (Rf Value)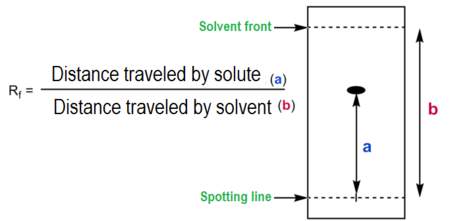
The movement of a substance in chromatography can be expressed using its Rf value:
\( \mathrm{Rf = \dfrac{Distance\ travelled\ by\ solute}{Distance\ travelled\ by\ solvent}} \)
- Each substance has a characteristic Rf value under fixed conditions (same solvent and paper).
- Rf values can be used to identify unknown substances by comparing them with known standards.
Example of Rf Calculation
If the solvent travels 10 cm up the paper and the blue dye spot travels 7 cm, then: ( \mathrm{Rf = \dfrac{7}{10} = 0.7} )
Applications of Chromatography
| Field | Application |
|---|---|
| Chemistry / Laboratory | Separation and identification of colored compounds or ions |
| Biology / Medicine | Separation of plant pigments, amino acids, and drugs |
| Forensic Science | Detection of inks, poisons, or narcotics in crime investigations |
| Environmental Science | Testing pollutants in air, water, and soil samples |
| Food Industry | Identifying artificial colors and preservatives in foods |
Advantages of Chromatography
- Requires only small sample quantities.
- Simple, quick, and inexpensive.
- Highly accurate and sensitive.
- Can separate complex mixtures into pure components.
- Used for both qualitative and quantitative analysis.
Example :
Why do different dyes in ink travel different distances on the same chromatogram?
▶️ Answer / Explanation
Step 1: Each dye has different solubility in the solvent and different attraction to the paper.
Step 2: Dyes that are more soluble move faster and farther up the paper.
Step 3: Dyes that are less soluble or more strongly attracted to paper move slower.
Final Answer: Dyes travel different distances because of differences in solubility and adsorption to the paper.
Example:
A green ink separates into yellow and blue spots after paper chromatography. What does this indicate about the ink’s composition?
▶️ Answer / Explanation
Step 1: The ink was a mixture of two dyes with different solubilities.
Step 2: The yellow dye moved faster (more soluble), and the blue dye moved slower (less soluble).
Final Answer: The green ink is a mixture of at least two colored substances — blue and yellow dyes.
Example :
Explain how chromatography could be used to test whether two samples of ink are from the same pen.
▶️ Answer / Explanation
Step 1: Place spots of both ink samples side by side on the same chromatogram and develop it in the same solvent.
Step 2: Observe and compare the number, color, and position (Rf values) of spots formed.
Step 3: If the spots match exactly, both samples are likely from the same pen; if not, they are different.
Final Answer: Chromatography identifies whether inks are identical by comparing their Rf values and spot patterns.