IB MYP 4-5 Chemistry -Collision theory and activation energy- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Collision theory and activation energy- Study Notes
Key Concepts
- Collision Theory and Activation Energy
Collision Theory and Activation Energy
Collision Theory and Activation Energy
The collision theory explains how and why chemical reactions occur. According to this theory, chemical reactions happen when reactant particles collide with each other with enough energy and the correct orientation.
Collision Theory — Main Concepts
For a reaction to occur, particles must:![]()
- Collide with one another.
- Collide with sufficient energy to overcome the activation energy barrier.
- Collide in the correct orientation so bonds can form and break properly.
Not all collisions result in reactions. Only a fraction of total collisions are successful these are called effective collisions.
![]()
Activation Energy (Eₐ)
The activation energy is the minimum amount of energy that reacting particles must have for a successful collision to result in a chemical reaction.
\( \mathrm{E_a = Minimum\ energy\ required\ for\ effective\ collision} \)![]()
- Low \( \mathrm{E_a} \): reaction happens easily and quickly.
- High \( \mathrm{E_a} \): reaction occurs slowly because few particles have enough energy.
Relation with Energy Profile Diagram:
- The peak of the energy curve represents the activated complex or transition state.
- The height from reactants to the peak = \( \mathrm{E_a} \).
- The difference between reactants and products = \( \mathrm{ΔH} \) (enthalpy change).
Energy Profile and Activation Energy
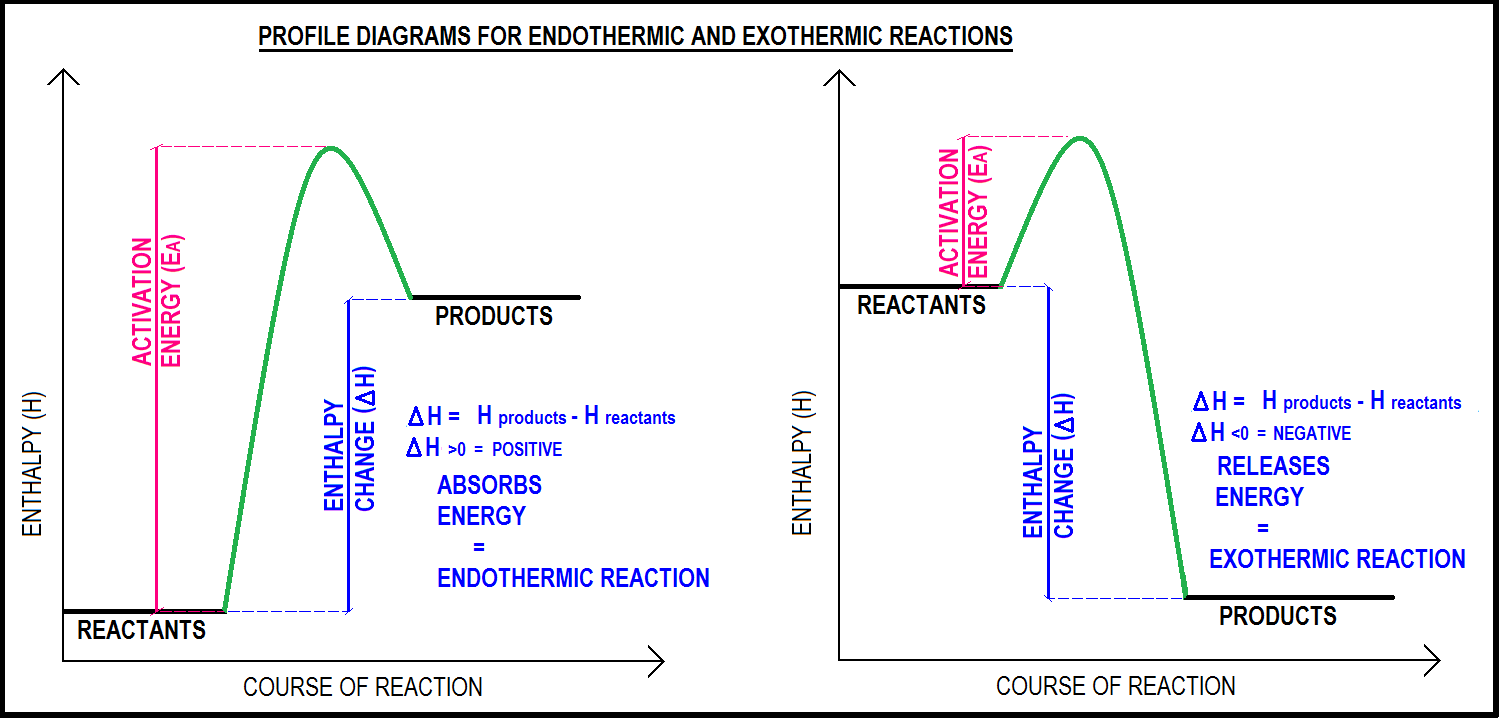
| Type of Reaction | Energy Flow | Activation Energy (Eₐ) | ΔH (Enthalpy Change) |
|---|---|---|---|
| Exothermic | Energy released | Small | Negative (ΔH < 0) |
| Endothermic | Energy absorbed | Large | Positive (ΔH > 0) |
Factors Affecting Collision Frequency and Success
- Temperature: Increases average kinetic energy → more particles have ≥ \( \mathrm{E_a} \).
- Concentration (or Pressure for gases): More particles per volume → more collisions per second.
- Surface Area: Greater area for collisions → higher frequency of effective collisions.
- Catalyst: Provides an alternative pathway with lower activation energy → increases rate of reaction.
The Role of Catalysts
A catalyst is a substance that increases the rate of a chemical reaction without being consumed, by providing an alternative reaction pathway with a lower activation energy.
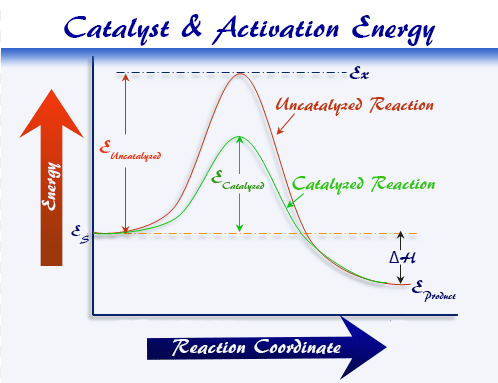
- Catalyst lowers \( \mathrm{E_a} \), allowing more particles to have sufficient energy for successful collisions.
- Does not change \( \mathrm{ΔH} \) of the reaction.
Maxwell–Boltzmann Distribution (Conceptual)
The energy of particles in a sample is distributed over a range of values. Only particles with energy greater than or equal to \( \mathrm{E_a} \) can react.
![]()
- Increasing temperature → shifts curve right → more particles ≥ \( \mathrm{E_a} \).
- Using a catalyst → lowers \( \mathrm{E_a} \) → increases fraction of successful collisions.
Summary Table: Collision Theory and Activation Energy
| Concept | Description | Effect on Reaction Rate |
|---|---|---|
| Collision Theory | Reactions occur when particles collide with sufficient energy and correct orientation. | Explains rate increase with temperature, concentration, and catalysts. |
| Activation Energy (Eₐ) | Minimum energy needed for reaction to start. | Lower \( \mathrm{E_a} \) → faster reaction. |
| Catalyst | Provides alternative pathway with lower \( \mathrm{E_a} \). | Increases reaction rate. |
Example
Why does increasing temperature increase the rate of a reaction?
▶️ Answer / Explanation
Step 1: Higher temperature → particles move faster → more frequent collisions.
Step 2: A greater proportion of particles have energy ≥ \( \mathrm{E_a} \).
Final Answer: The reaction rate increases because more collisions are effective.
Example
Explain why a catalyst increases the rate of a chemical reaction without being consumed.
▶️ Answer / Explanation
Step 1: Catalyst provides an alternative pathway with lower activation energy.
Step 2: More reactant particles now have sufficient energy to react.
Step 3: Catalyst remains chemically unchanged at the end of the reaction.
Final Answer: The catalyst speeds up the reaction by lowering \( \mathrm{E_a} \) but is not consumed.
Example
Two reactions have activation energies of 50 kJ/mol and 150 kJ/mol. Which reaction is faster at room temperature and why?
▶️ Answer / Explanation
Step 1: The reaction with lower activation energy (50 kJ/mol) requires less energy for collisions to be effective.
Step 2: At room temperature, a greater fraction of particles have enough energy to overcome the 50 kJ barrier.
Final Answer: The reaction with \( \mathrm{E_a = 50\ kJ/mol} \) is faster because more particles have sufficient energy for effective collisions.
