IB MYP 4-5 Chemistry -Corrosion and methods of prevention- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Corrosion and methods of prevention- Study Notes
Key Concepts
- Corrosion and Methods of Prevention
Corrosion and Methods of Prevention
Corrosion and Methods of Prevention
Corrosion is a slow, natural process in which metals are gradually oxidized by air, moisture, or other chemicals, forming undesired compounds such as oxides, hydroxides, or carbonates on their surface. It is an example of a redox reaction where the metal acts as the reducing agent and is itself oxidized.
Corrosion weakens metals, changes their appearance, and leads to loss of material strength and economic damage.
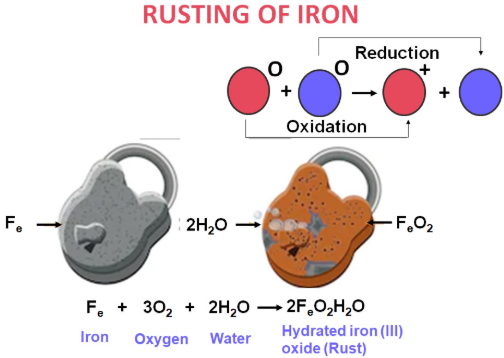
Common Example — Rusting of Iron
Rusting is the corrosion of iron when exposed to oxygen and water. The reddish-brown flaky substance formed is hydrated iron(III) oxide, \( \mathrm{Fe_2O_3 \cdot xH_2O} \).
Chemical Reactions Involved:
- Oxidation at anodic region: \( \mathrm{Fe \rightarrow Fe^{2+} + 2e^-} \)
- Reduction at cathodic region: \( \mathrm{O_2 + 2H_2O + 4e^- \rightarrow 4OH^-} \)
- Formation of rust: \( \mathrm{Fe^{2+} + OH^- + O_2 \rightarrow Fe_2O_3 \cdot xH_2O} \)
Conditions Required for Rusting:
- Presence of oxygen (from air)
- Presence of water or moisture
- Presence of electrolytes (e.g., salts, acids) accelerates rusting
Types of Corrosion
Corrosion can occur in different forms depending on environmental conditions, the type of metal, and exposure to electrolytes. Below are the main types of corrosion and their mechanisms:

| Type of Corrosion | Mechanism / Description | Example |
|---|---|---|
| 1. Uniform Corrosion | Occurs evenly across the surface of a metal when exposed uniformly to air and moisture. The entire surface reacts at nearly the same rate, leading to gradual thinning. | Rusting of iron sheets exposed to rain |
| 2. Galvanic Corrosion | Occurs when two dissimilar metals are connected in the presence of an electrolyte. The more reactive metal (anode) corrodes faster, while the less reactive one (cathode) is protected. | Iron and copper in saltwater — iron corrodes |
| 3. Pitting Corrosion | A localized form of corrosion that creates small pits or holes. Often caused by breakdown of a protective oxide layer on the metal surface. | Aluminium or stainless steel exposed to chloride ions |
| 4. Crevice Corrosion | Occurs in confined spaces or crevices (like joints, bolts, or gaskets) where oxygen concentration differs. The lower-oxygen region acts as the anode and corrodes faster. | Corrosion under washers or gaskets in ships |
| 5. Stress Corrosion Cracking (SCC) | Occurs due to combined action of mechanical stress and a corrosive environment. Leads to cracks forming in metals even under moderate conditions. | Brass fittings under tension in ammonia atmosphere |
Note: Galvanic and crevice corrosion are examples of electrochemical corrosion, where anodic and cathodic regions are formed on or between metals, leading to redox reactions and metal oxidation.
Methods of Preventing Corrosion
Corrosion can be controlled or slowed using both physical and chemical methods:
| Method | Description | Example / Reaction |
|---|---|---|
| Painting or Coating | Creates a physical barrier between metal and air/moisture. | Automobile body paints, enamel coating |
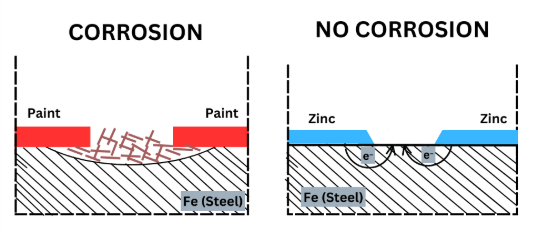
| Galvanization | Iron coated with a thin layer of zinc. Zinc acts as sacrificial metal. | \( \mathrm{Zn \rightarrow Zn^{2+} + 2e^-} \) |
| Tin Plating | Tin layer protects iron but if scratched, corrosion occurs faster (tin less reactive). | Food cans |
| Alloying | Mixing metals increases corrosion resistance (due to protective oxide layers). | Stainless steel = Iron + Chromium + Nickel |
| Cathodic Protection | Connecting iron to a more reactive metal (e.g., Mg or Zn) makes iron act as cathode. | Used for pipelines and ships |
| Oil or Grease Coating | Prevents direct contact with air and water. | Machinery and tools |
Role of Redox in Corrosion Prevention
- Corrosion involves oxidation of metal (loss of electrons).
- In galvanization or cathodic protection, a more reactive metal (like Zn or Mg) oxidizes first, protecting the main metal from oxidation.
- Thus, prevention strategies involve controlling the redox process by altering electron flow or blocking reactants.
Example:
Why does rusting of iron occur faster in coastal regions?
▶️ Answer / Explanation
Step 1: Moist air and high salt concentration increase conductivity.
Step 2: Presence of electrolytes accelerates electron transfer in redox reactions.
Final Answer: Rusting is faster in coastal regions due to high humidity and salt ions that promote redox reactions.
Example:
Explain how zinc protects iron in galvanized steel even if the surface is scratched.
▶️ Answer / Explanation
Step 1: Zinc is more reactive than iron and acts as a sacrificial metal.
Step 2: When exposed, zinc preferentially oxidizes:
\( \mathrm{Zn \rightarrow Zn^{2+} + 2e^-} \)
Step 3: Electrons from zinc prevent iron from oxidizing.
Final Answer: Zinc sacrifices itself (corrodes) to protect iron — a redox-based protection.
Example:
A steel pipeline is buried underground and connected to a magnesium block. Explain why this setup prevents corrosion of the pipeline and what type of protection it represents.
▶️ Answer / Explanation
Step 1: Magnesium is more reactive than iron (higher in reactivity series).
Step 2: Magnesium acts as an anode, oxidizing first:
\( \mathrm{Mg \rightarrow Mg^{2+} + 2e^-} \)
Step 3: Electrons flow to the steel pipeline, making it the cathode (protected from oxidation).
Final Answer: This is cathodic protection using a sacrificial anode; magnesium corrodes instead of the steel pipeline.
