IB MYP 4-5 Chemistry -Diffusion and Brownian motion- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Diffusion and Brownian motion- Study Notes
Key Concepts
- Diffusion
- Brownian Motion and Evidence for Particle Movement
Diffusion
Diffusion
Diffusion is the spontaneous movement of particles from an area of higher concentration to an area of lower concentration, until they are evenly distributed. It occurs because particles are in constant random motion.
Understanding Diffusion
Diffusion is an example of how particles move due to their natural kinetic energy. No external force or stirring is required the process happens automatically in gases and liquids.
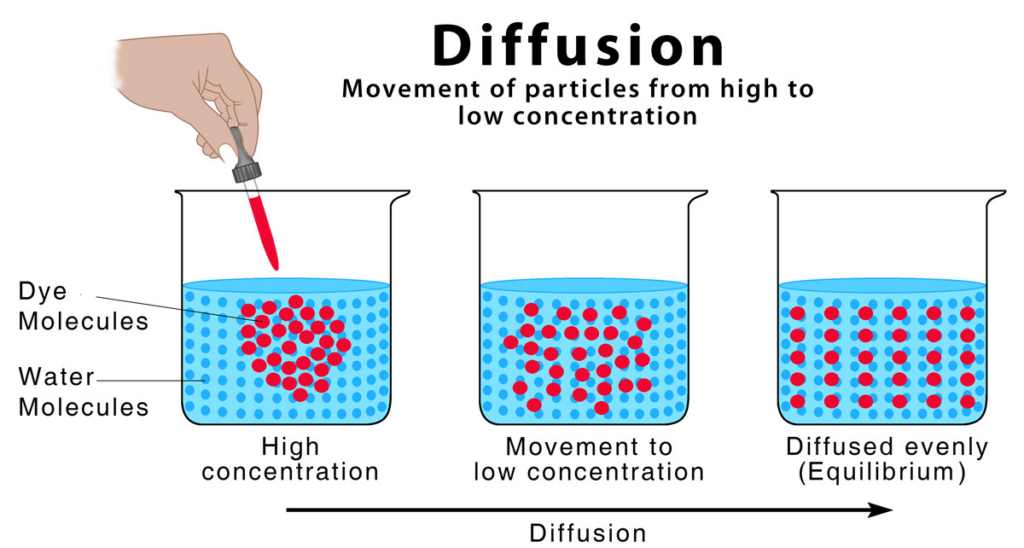
- Particles move randomly and collide with each other.
- Over time, these random movements spread the particles throughout the available space.
- Diffusion continues until a uniform concentration (equilibrium) is reached.
Factors Affecting Diffusion Rate
| Factor | Effect on Diffusion | Explanation |
|---|---|---|
| Temperature | Higher temperature → faster diffusion | Particles move faster at higher temperature because their kinetic energy increases. |
| Particle Mass | Lighter particles diffuse faster | Lighter particles have higher speeds for the same amount of energy. |
| Medium (state of matter) | Fastest in gases, slower in liquids, very slow in solids | Particle spacing and mobility are greatest in gases and smallest in solids. |
Diffusion in Different States of Matter
- In gases: Diffusion is fastest because gas particles move freely and rapidly with little resistance.
- In liquids: Diffusion is slower because particles are closer and experience more collisions.
- In solids: Diffusion is extremely slow or negligible because particles vibrate in fixed positions.
Everyday Examples of Diffusion
- Smell of perfume spreading in a room.
- Food coloring mixing in water without stirring.
- Oxygen and carbon dioxide exchange in the lungs.
- Sugar dissolving and spreading evenly through tea.
Example :
Why does the smell of perfume spread quickly across a warm room but slowly in a cold room?
▶️ Answer / Explanation
Step 1: Diffusion rate depends on the speed of moving particles.
Step 2: At higher temperature, air particles move faster because they have more kinetic energy.
Step 3: The perfume molecules collide and spread more rapidly through the air.
Final Answer: Perfume spreads faster in a warm room because higher temperature increases particle motion, speeding up diffusion.
Example :
When a crystal of potassium permanganate (\( \mathrm{KMnO_4} \)) is placed in still water, the purple color gradually spreads throughout the water. Explain this process in terms of diffusion.
▶️ Answer / Explanation
Step 1: The crystal slowly dissolves, releasing \( \mathrm{KMnO_4} \) particles into the surrounding water.
Step 2: These particles move from regions of higher concentration (near the crystal) to lower concentration (rest of the water).
Step 3: The movement continues until the color becomes evenly distributed.
Final Answer: The purple color spreads by diffusion — the random motion of particles from concentrated to less concentrated regions.
Example :
Explain why diffusion of gases occurs much faster than diffusion of liquids, using only macroscopic reasoning (no particle-level explanation).
▶️ Answer / Explanation
Step 1: Gases occupy larger volumes and expand easily, while liquids are more compact and resist flow.
Step 2: Because gases spread to fill the entire space quickly, the mixing of one gas into another happens almost instantly.
Step 3: Liquids, being denser, require more time for uniform mixing because their flow is slower and more restricted.
Final Answer: Diffusion in gases is faster because gases expand freely and move rapidly throughout the available space, while liquids flow more slowly.
Brownian Motion and Evidence for Particle Movement
Brownian Motion and Evidence for Particle Movement
Brownian motion is the random, zigzag movement of tiny particles suspended in a fluid (liquid or gas). It provides strong experimental evidence that all matter is made of constantly moving particles.
Discovery of Brownian Motion
In 1827, the botanist Robert Brown observed under a microscope that pollen grains suspended in water moved in a continuous, irregular motion. He found that this movement did not stop and was not caused by life — it happened with all small particles, even non-living dust or soot.
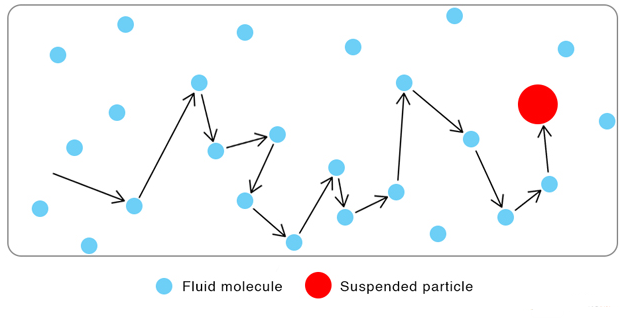
This phenomenon became known as Brownian motion.
Cause of Brownian Motion
Brownian motion happens because the suspended particles are continuously bombarded by much smaller, fast-moving particles of the surrounding fluid.
- In a liquid → water molecules collide with pollen or dust particles.
- In a gas → air molecules strike small particles like smoke or dust.
- These collisions are uneven and random, causing the visible, jerky motion.
Evidence for the Particle Theory of Matter
Brownian motion supports the particle model of matter because it shows that:
- Matter is made up of tiny, invisible particles.
- These particles are in constant, random motion.
- They possess kinetic energy, which increases with temperature.
Effect of Temperature on Brownian Motion
![]()
- At higher temperature → fluid particles move faster → suspended particles move more vigorously.
- At lower temperature → collisions are slower and gentler → motion is less noticeable.
Examples and Experiments Demonstrating Brownian Motion
| Example / Experiment | Medium | Observation | Explanation |
|---|---|---|---|
| Pollen grains in water (Robert Brown) | Liquid | Grains move randomly in zigzag paths | Collisions with moving water molecules cause the motion. |
| Smoke particles under microscope | Gas (air) | Tiny smoke particles move irregularly | Air molecules collide randomly with the smoke particles. |
| Dust particles floating in sunlight | Gas (air) | Dust appears to dance or shimmer in the light | Air molecules in motion push the dust particles randomly. |
Note:
- Brownian motion provides visible proof that particles of matter move constantly.
- It demonstrates that even seemingly still substances (like air or water) contain moving particles.
- The motion becomes more vigorous at higher temperatures due to increased particle energy.
Example :
Explain how Brownian motion provides evidence for the kinetic theory of matter.
▶️ Answer / Explanation
Step 1: The kinetic theory states that all matter is made of particles that move constantly.
Step 2: In Brownian motion, the visible particles move randomly due to collisions with invisible, faster-moving particles of the medium.
Step 3: This shows that matter consists of moving particles with kinetic energy, confirming the kinetic theory.
Final Answer: Brownian motion proves that particles of matter are in continuous motion and possess kinetic energy, as described by the kinetic theory.
Example :
When smoke particles are viewed under a microscope through a bright beam of light, they are seen to move in a zigzag path. What causes this motion and how does it change with temperature?
▶️ Answer / Explanation
Step 1: Smoke particles are hit unevenly by air molecules moving in random directions.
Step 2: These unequal collisions push the smoke particles in irregular, zigzag paths — Brownian motion.
Step 3: As temperature increases, air molecules move faster and collide more vigorously, making the smoke move faster.
Final Answer: The zigzag motion is caused by uneven collisions with air molecules, and the motion becomes faster as temperature increases.
Example:
Explain how Brownian motion supports the idea that gases exert pressure, using macroscopic reasoning.
▶️ Answer / Explanation
Step 1: Brownian motion shows that particles in a gas are moving and colliding continuously.
Step 2: In a container, these continuous collisions occur not just with suspended particles but also with the container walls.
Step 3: Each collision exerts a tiny force; the total effect of many collisions produces measurable gas pressure.
Final Answer: Brownian motion supports the idea that gases exert pressure because it visually demonstrates that gas particles move randomly and collide continuously with surfaces.
