IB MYP 4-5 Chemistry -Electrochemical cells- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Electrochemical cells- Study Notes
Key Concepts
- Electrochemical Cells
- Electrolysis and Its Applications (Electroplating, Extraction, and Purification)
Electrochemical Cells
Electrochemical Cells
An electrochemical cell is a device that converts chemical energy into electrical energy (or vice versa) through redox (reduction–oxidation) reactions.
Basic Principle
Electrochemical cells are based on the movement of electrons from one substance to another during a redox reaction:
- Oxidation occurs at the anode (loss of electrons).

- Reduction occurs at the cathode (gain of electrons).
Key Equation:
\( \mathrm{Redox\ Reaction = Oxidation + Reduction} \)
As electrons move from the anode to the cathode through an external circuit, an electric current is generated.
Types of Electrochemical Cells
There are two main types of electrochemical cells:
| Type of Cell | Energy Conversion | Description | Example |
|---|---|---|---|
| Galvanic (Voltaic) Cell | Chemical → Electrical | Spontaneous redox reactions produce electric current. | Daniell cell (Zn–Cu) |
| Electrolytic Cell | Electrical → Chemical | Non-spontaneous reactions are driven by an external power source. | Electrolysis of water, electroplating |
Structure of a Galvanic (Voltaic) Cell
Example: Daniell Cell (Zn–Cu)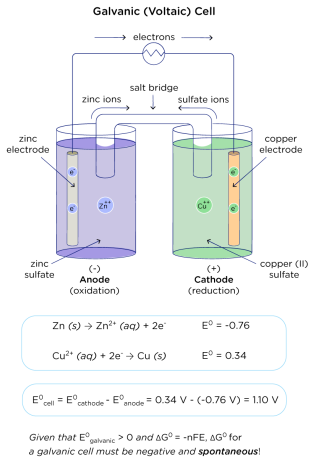
- Anode: Zinc (Zn) electrode in ZnSO₄ solution → oxidation
- Cathode: Copper (Cu) electrode in CuSO₄ solution → reduction
- Salt Bridge: Allows ion flow to maintain charge balance
- External Circuit: Path for electron flow from Zn → Cu
Overall Reaction:
\( \mathrm{Zn(s) + Cu^{2+}(aq) \rightarrow Zn^{2+}(aq) + Cu(s)} \)
At Electrodes:
- Anode (oxidation): \( \mathrm{Zn(s) \rightarrow Zn^{2+}(aq) + 2e^-} \)
- Cathode (reduction): \( \mathrm{Cu^{2+}(aq) + 2e^- \rightarrow Cu(s)} \)
Electron Flow: From zinc (anode) → copper (cathode)
Salt Bridge Function:
- Maintains electrical neutrality by allowing ions to move:
- \( \mathrm{NO_3^-} \) ions move toward the anode compartment.
- \( \mathrm{K^+} \) ions move toward the cathode compartment.
Energy Conversion in a Galvanic Cell
In a galvanic cell:
- Chemical energy from a spontaneous redox reaction is converted to electrical energy.
- Voltage (cell potential) depends on the reactivity difference between the two metals.
Cell Notation:
![]()
Electrolytic Cells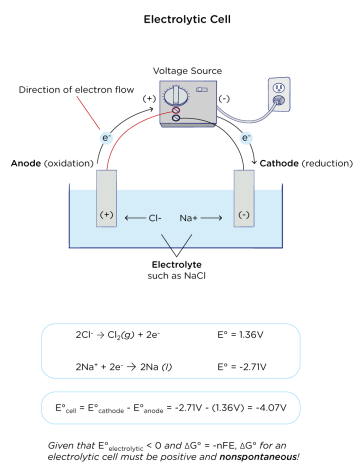
An electrolytic cell uses an external power source to drive a non-spontaneous chemical reaction.
- Electrons are forced to flow from anode to cathode by an external battery.
- Anode: Positive (oxidation)
- Cathode: Negative (reduction)
Example: Electrolysis of Water
\( \mathrm{2H_2O(l) \rightarrow 2H_2(g) + O_2(g)} \)
- Anode (oxidation): \( \mathrm{2H_2O \rightarrow O_2 + 4H^+ + 4e^-} \)
- Cathode (reduction): \( \mathrm{4H_2O + 4e^- \rightarrow 2H_2 + 4OH^-} \)
Comparison: Galvanic vs Electrolytic Cell
| Feature | Galvanic (Voltaic) Cell | Electrolytic Cell |
|---|---|---|
| Energy Conversion | Chemical → Electrical | Electrical → Chemical |
| Reaction Type | Spontaneous | Non-spontaneous |
| Anode Polarity | Negative | Positive |
| Electron Flow | Anode → Cathode | Power source forces Anode → Cathode |
Example
In a Daniell cell, which electrode acts as the anode and which as the cathode?
▶️ Answer / Explanation
Step 1: Zinc is oxidized, producing \( \mathrm{Zn^{2+}} \) ions and electrons.
Step 2: Copper gains electrons and is reduced.
Final Answer: Anode: Zinc (oxidation) Cathode: Copper (reduction)
Example
Write the half-equations and overall reaction for a galvanic cell using magnesium and copper electrodes in their respective sulfate solutions.
▶️ Answer / Explanation
Step 1: Mg is more reactive than Cu → Mg is oxidized, Cu²⁺ is reduced.
At anode (oxidation): \( \mathrm{Mg(s) \rightarrow Mg^{2+}(aq) + 2e^-} \)
At cathode (reduction): \( \mathrm{Cu^{2+}(aq) + 2e^- \rightarrow Cu(s)} \)
Overall: \( \mathrm{Mg(s) + Cu^{2+}(aq) \rightarrow Mg^{2+}(aq) + Cu(s)} \)
Final Answer: Magnesium acts as the anode and copper as the cathode.
Example
In an electrolytic cell used for electroplating silver onto a spoon, identify the electrodes and write the half-equations.
▶️ Answer / Explanation
Step 1: Silver is the metal being deposited → silver acts as cathode source.
Step 2: The spoon (object to be plated) is the cathode.
At anode (oxidation): \( \mathrm{Ag(s) \rightarrow Ag^{+}(aq) + e^-} \)
At cathode (reduction): \( \mathrm{Ag^{+}(aq) + e^- \rightarrow Ag(s)} \)
Final Answer: The spoon is the cathode (gains silver), the silver metal plate is the anode (dissolves).
Electrolysis and Its Applications (Electroplating, Extraction, and Purification)
Electrolysis and Its Applications (Electroplating, Extraction, and Purification)
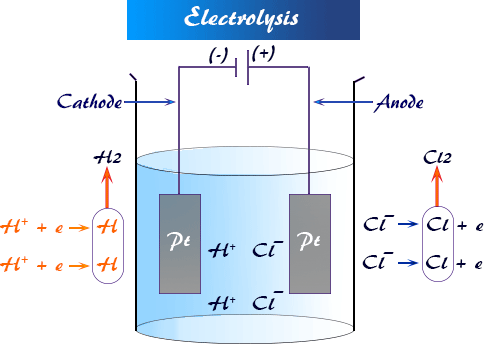
Electrolysis is the process in which electrical energy is used to drive a non-spontaneous chemical reaction. It occurs in an electrolytic cell, where electricity causes chemical changes in an ionic substance.
The Electrolytic Cell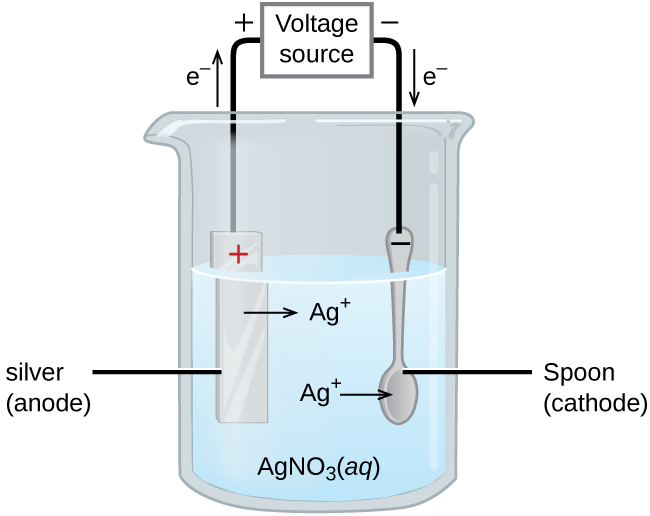
Components:
- Electrolyte: The ionic substance (molten or in solution) that conducts electricity.
- Anode (Positive Electrode): Site of oxidation — electrons are released.
- Cathode (Negative Electrode): Site of reduction — electrons are gained.
- Power Supply: Provides external electrical energy to drive the reaction.
Electron Flow: From the power supply to the cathode and then through the electrolyte to the anode.
Process of Electrolysis
Step-by-step Process:
- When electricity passes through the electrolyte, ions move.
- Positive ions (cations) move to the cathode and gain electrons (reduction).

- Negative ions (anions) move to the anode and lose electrons (oxidation).
- New substances are formed at both electrodes.
General Reactions:
- At Cathode (Reduction): \( \mathrm{M^{n+} + ne^- \rightarrow M(s)} \)
- At Anode (Oxidation): \( \mathrm{X^- \rightarrow X + e^-} \)
Example: Electrolysis of Molten Sodium Chloride (NaCl)
![]()
- Electrolyte: Molten NaCl
- At Cathode: \( \mathrm{Na^+ + e^- \rightarrow Na(s)} \)
- At Anode: \( \mathrm{2Cl^- \rightarrow Cl_2(g) + 2e^-} \)
- Overall Reaction: \( \mathrm{2NaCl(l) \rightarrow 2Na(s) + Cl_2(g)} \)
Products: Sodium metal (at cathode) and chlorine gas (at anode).
Applications of Electrolysis
Electrolysis has several industrial and laboratory applications, including:
- Electroplating: Coating an object with a thin layer of another metal.
- Extraction of Metals: Obtaining pure metals from molten ores (e.g., aluminium from bauxite).
- Purification of Metals: Removing impurities from impure metals (e.g., copper refining).
Electroplating
Electroplating is the process of coating an object (usually metal) with a thin layer of another metal using electrolysis. 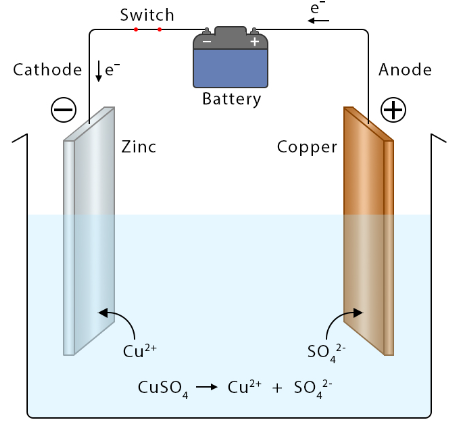
Purpose:
- To improve appearance (e.g., silver plating jewelry).
- To prevent corrosion (e.g., chrome-plated car parts).
Setup:
- Anode: Metal to be plated (e.g., silver).
- Cathode: Object to be coated (e.g., spoon).
- Electrolyte: Solution of a salt of the plating metal (e.g., AgNO₃).
Reactions (Silver Plating Example):
- Anode: \( \mathrm{Ag(s) \rightarrow Ag^+ + e^-} \)
- Cathode: \( \mathrm{Ag^+ + e^- \rightarrow Ag(s)} \)
Extraction of Metals (Example: Aluminium)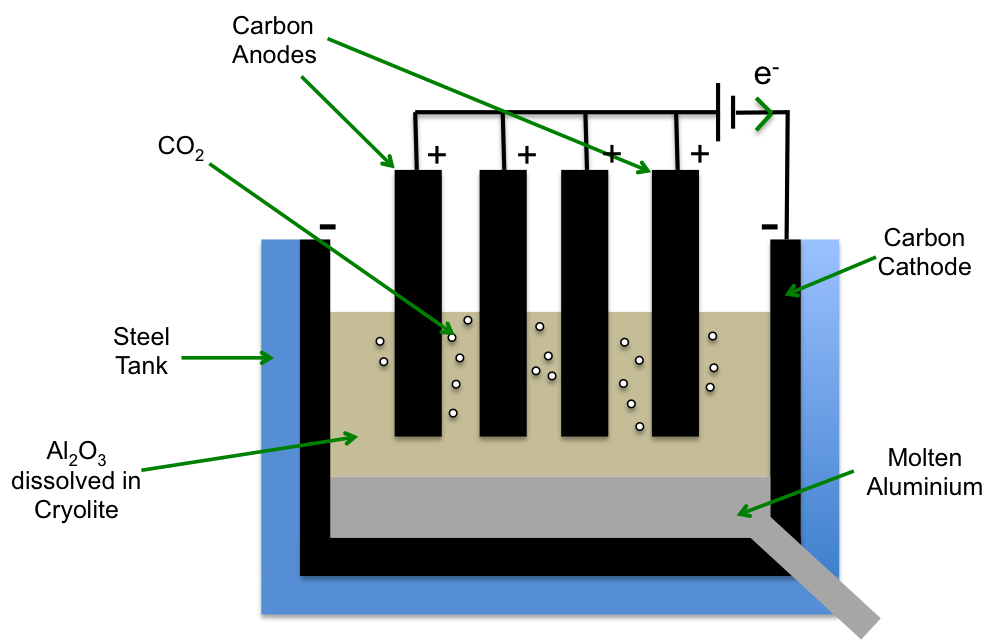
Process: Electrolysis of molten aluminium oxide (Al₂O₃) in the Hall–Héroult process.
- Electrolyte: Molten Al₂O₃ dissolved in cryolite (Na₃AlF₆) to lower melting point.
- Cathode: \( \mathrm{Al^{3+} + 3e^- \rightarrow Al(l)} \)
- Anode: \( \mathrm{2O^{2-} \rightarrow O_2(g) + 4e^-} \)
- Overall: \( \mathrm{2Al_2O_3 \rightarrow 4Al + 3O_2} \)
Uses: Extraction of reactive metals like aluminium and magnesium.
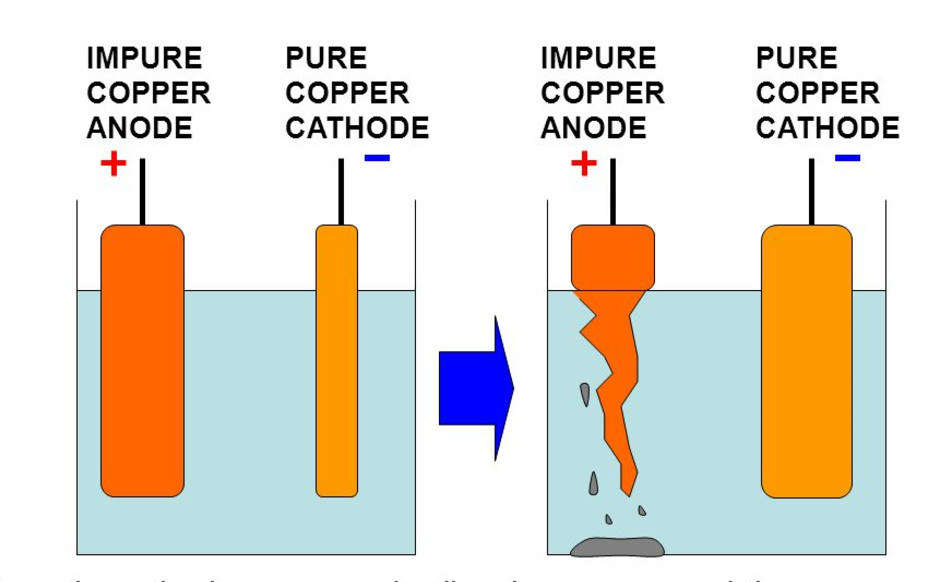
Purification of Metals (Example: Copper Refining)
Purpose: To obtain pure copper from impure copper metal using electrolysis.
- Anode: Impure copper (Cu).

- Cathode: Pure copper sheet.
- Electrolyte: Copper(II) sulfate solution (\( \mathrm{CuSO_4} \)).
Reactions:
- Anode: \( \mathrm{Cu(s) \rightarrow Cu^{2+} + 2e^-} \)
- Cathode: \( \mathrm{Cu^{2+} + 2e^- \rightarrow Cu(s)} \)
Result: Pure copper is deposited at the cathode; impurities fall off as sludge.
Summary Table: Electrolysis Applications
| Application | Example | Anode | Cathode | Electrolyte |
|---|---|---|---|---|
| Electroplating | Silver plating | Silver | Spoon/object | AgNO₃ solution |
| Extraction | Aluminium (Al₂O₃) | Graphite (C) | Molten Al₂O₃ | Cryolite + Al₂O₃ |
| Purification | Copper Refining | Impure Cu | Pure Cu | CuSO₄ solution |
Example
During the electrolysis of molten NaCl, what products are formed at each electrode?
▶️ Answer / Explanation
Step 1: Na⁺ and Cl⁻ ions are present in molten NaCl.
Step 2: At cathode → \( \mathrm{Na^+ + e^- \rightarrow Na(s)} \)
Step 3: At anode → \( \mathrm{2Cl^- \rightarrow Cl_2(g) + 2e^-} \)
Final Answer: Sodium metal at cathode, chlorine gas at anode.
Example
In the electrolysis of copper(II) sulfate solution using copper electrodes, which electrode gains mass and why?
▶️ Answer / Explanation
Step 1: At anode → \( \mathrm{Cu(s) \rightarrow Cu^{2+} + 2e^-} \)
Step 2: At cathode → \( \mathrm{Cu^{2+} + 2e^- \rightarrow Cu(s)} \)
Step 3: Copper dissolves from anode and deposits on cathode.
Final Answer: The cathode gains mass as pure copper is deposited.
Example
Explain why aluminium oxide must be dissolved in cryolite before electrolysis, and describe the reactions at each electrode.
▶️ Answer / Explanation
Step 1: Pure Al₂O₃ has a very high melting point (~2050°C).
Step 2: Cryolite lowers the melting point and improves conductivity.
Step 3: At cathode → \( \mathrm{Al^{3+} + 3e^- \rightarrow Al(l)} \)
Step 4: At anode → \( \mathrm{2O^{2-} \rightarrow O_2(g) + 4e^-} \)
Final Answer: Aluminium is deposited at the cathode and oxygen gas is released at the anode. Cryolite allows the process to occur efficiently at a lower temperature.