IB MYP 4-5 Chemistry -Electron configuration and valency- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Electron configuration and valency- Study Notes
Key Concepts
- Electron Configuration and Valency
Electron Configuration and Valency
Electron Configuration
Atoms contain electrons arranged in specific energy levels or shells around the nucleus. The arrangement of these electrons in different shells is called the electron configuration.
Key Concept: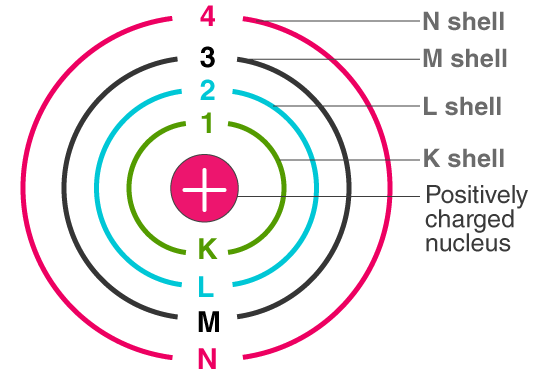
Electron configuration determines how atoms bond and react it directly influences an atom’s valency.
Structure of an Atom
- Electrons move around the nucleus in fixed energy levels called shells.
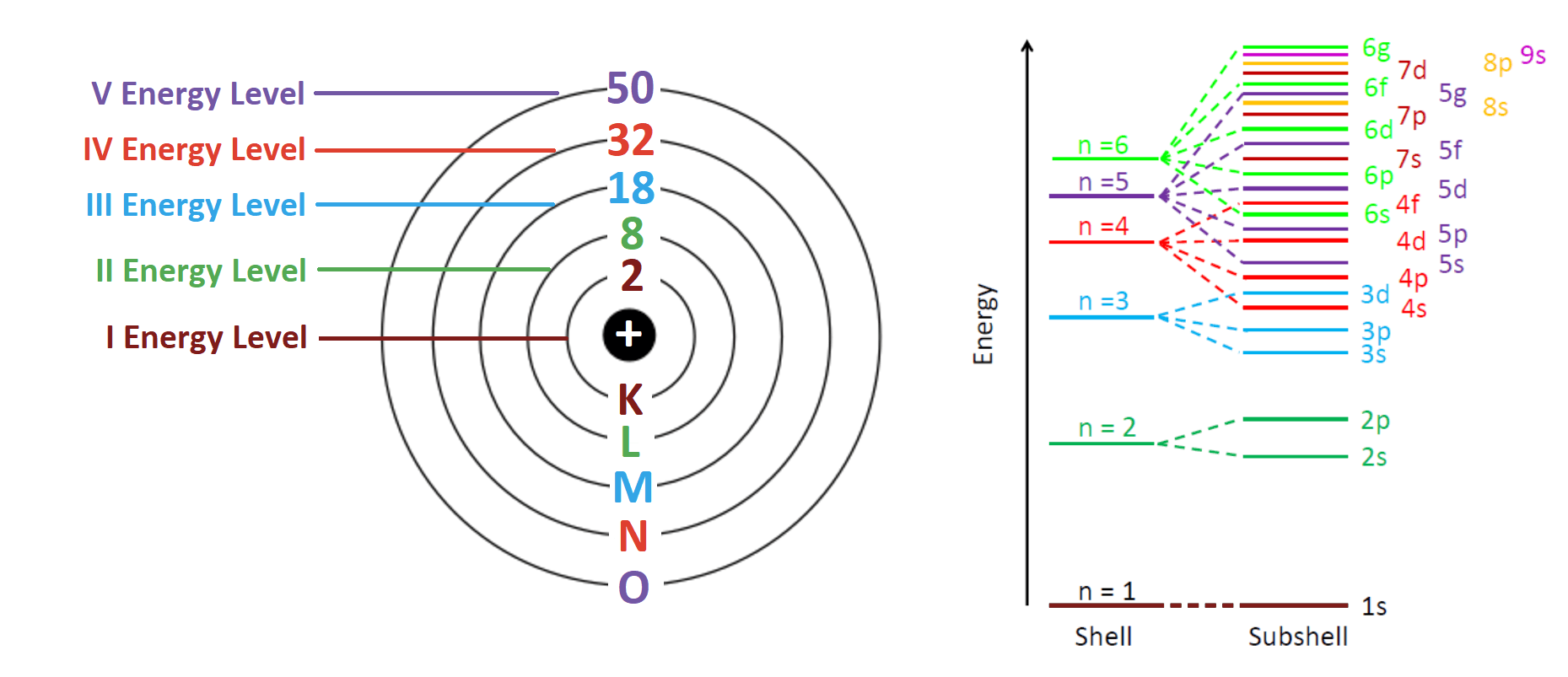
- Each shell can hold a fixed maximum number of electrons:
\( \mathrm{Maximum\ electrons\ in\ a\ shell = 2n^2} \)
where \( \mathrm{n} \) = shell number (1, 2, 3, …)
| Shell | Symbol | Maximum Electrons |
|---|---|---|
| 1st Shell | K | 2 |
| 2nd Shell | L | 8 |
| 3rd Shell | M | 18 |
| 4th Shell | N | 32 |
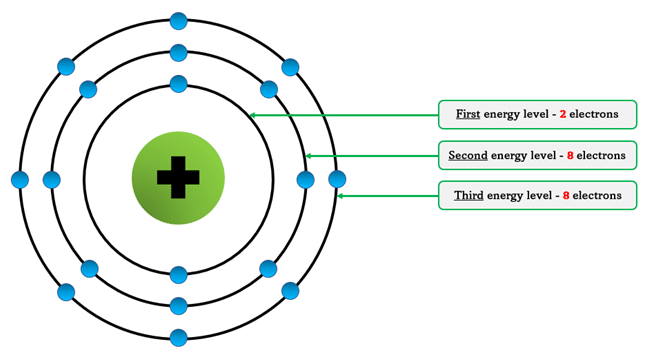
Rules for Writing Electron Configuration
- Electrons fill the lowest energy level first (closest to the nucleus).
- Maximum electrons per shell follow the 2, 8, 8, 18 pattern (simplified for first 20 elements).
- When one shell is full, the next one begins to fill.
Example Elements:
| Element | Atomic Number | Electron Configuration | Shell Distribution |
|---|---|---|---|
| Hydrogen (H) | 1 | 1 | K = 1 |
| Carbon (C) | 6 | 2, 4 | K = 2, L = 4 |
| Oxygen (O) | 8 | 2, 6 | K = 2, L = 6 |
| Sodium (Na) | 11 | 2, 8, 1 | K = 2, L = 8, M = 1 |
Key Idea:
The outermost shell (also called the valence shell) determines how an atom reacts. Electrons in this shell are called valence electrons.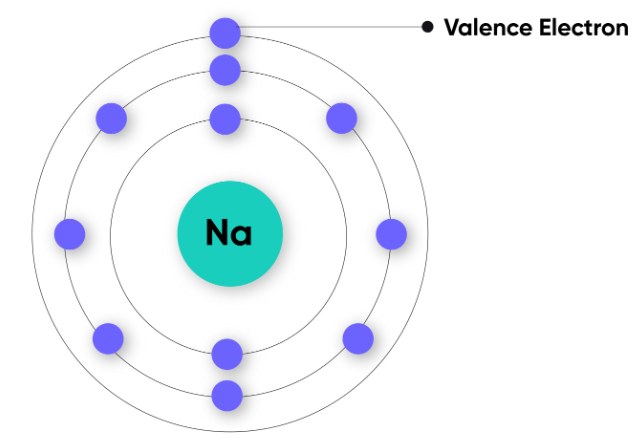
Example: Sodium (Na) → electron configuration = 2,8,1 → 1 valence electron.
Valency
Valency is the combining power of an atom it shows how many electrons an atom gains, loses, or shares to form a stable compound.
- Atoms try to achieve a full outer shell (octet rule).
- Metals usually lose electrons → positive valency.
- Non-metals usually gain electrons → negative valency.
\( \mathrm{Valency = Number\ of\ electrons\ lost,\ gained,\ or\ shared} \)
Examples of Valency
| Element | Electron Configuration | Valence Electrons | Valency | Tendency |
|---|---|---|---|---|
| Hydrogen (H) | 1 | 1 | 1 | Loses 1 or shares 1 |
| Oxygen (O) | 2, 6 | 6 | 2 | Gains 2 electrons |
| Sodium (Na) | 2, 8, 1 | 1 | 1 | Loses 1 electron |
| Chlorine (Cl) | 2, 8, 7 | 7 | 1 | Gains 1 electron |
Key Relationships
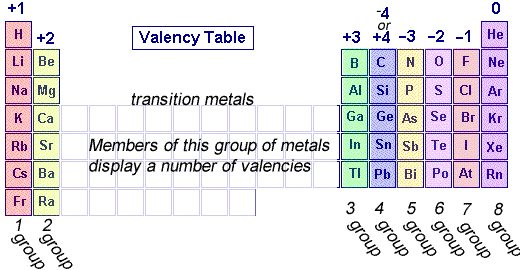
- Valency is related to the number of electrons in the outermost shell.
- Elements in the same group of the periodic table have the same valency.
- For first 20 elements, valency = number of outer electrons (if ≤ 4), or (8 − outer electrons) if > 4.
Example: Oxygen (6 outer electrons) → Valency = \( \mathrm{8 – 6 = 2} \)
Example :
Find the electron configuration and valency of magnesium (atomic number 12).
▶️ Answer / Explanation
Step 1: Atomic number = 12 → 12 electrons.
Step 2: Electron configuration = 2, 8, 2.
Step 3: Valence electrons = 2 → atom loses 2 to become stable.
Final Answer: Valency = 2.
Example :
Explain why elements in the same group of the periodic table have similar chemical properties.
▶️ Answer / Explanation
Step 1: Elements in the same group have the same number of valence electrons.
Step 2: Valence electrons determine bonding and chemical reactions.
Final Answer: Hence, elements in the same group show similar chemical behavior because they have identical valence electron arrangements.
