IB MYP 4-5 Chemistry -Energy profiles and bond energy- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Energy profiles and bond energy- Study Notes
Key Concepts
- Energy Profiles and Bond Energy
Energy Profiles and Bond Energy
Energy Profiles and Bond Energy
During a chemical reaction, bonds in the reactants are broken and new bonds are formed in the products. This process involves changes in energy, represented by energy profile diagrams and calculated using bond energies.
Energy Profile Diagrams
An energy profile diagram (or reaction energy diagram) shows how the energy of a system changes during a chemical reaction.
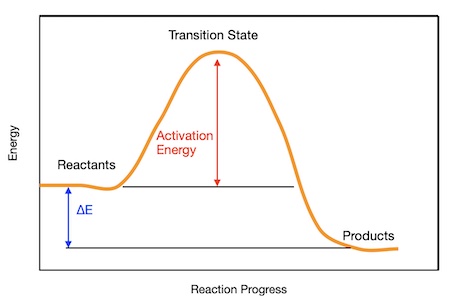
- It plots energy on the y-axis against reaction progress on the x-axis.
- It helps visualize the difference in energy between reactants and products and the activation energy (Eₐ).
Key Features of Energy Profiles:
- Reactants: Starting energy level.
- Products: Final energy level after reaction.
- Activation Energy (Eₐ): Minimum energy needed to start the reaction.
- ΔH (enthalpy change): Difference between the energy of products and reactants.
Exothermic and Endothermic Energy Profiles
![]()
| Reaction Type | Energy Change | ΔH Sign | Diagram Description |
|---|---|---|---|
| Exothermic | Energy released to surroundings | \( \mathrm{ΔH < 0} \) | Products have lower energy than reactants. |
| Endothermic | Energy absorbed from surroundings | \( \mathrm{ΔH > 0} \) | Products have higher energy than reactants. |
Formula for Enthalpy Change:![]()
\( \mathrm{ΔH = H_{products} – H_{reactants}} \)
- If \( \mathrm{ΔH < 0} \) → exothermic reaction.
- If \( \mathrm{ΔH > 0} \) → endothermic reaction.
Bond Energy
The bond energy (or bond enthalpy) is the amount of energy required to break one mole of a specific bond in a gaseous molecule.
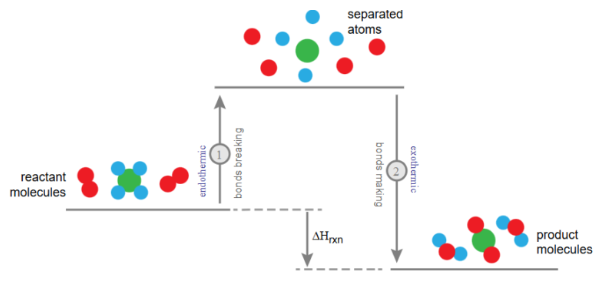
Symbol: \( \mathrm{E_{bond}} \) (measured in kJ/mol)
Key Idea:
- Breaking bonds → absorbs energy (endothermic process)
- Forming bonds → releases energy (exothermic process)
Formula for Overall Energy Change:
\( \mathrm{ΔH = Σ(Bond\ Energies\ of\ Bonds\ Broken) – Σ(Bond\ Energies\ of\ Bonds\ Formed)} \)
- If \( \mathrm{ΔH} \) is negative → reaction is exothermic.
- If \( \mathrm{ΔH} \) is positive → reaction is endothermic.
Example of Bond Energy Values
| Bond | Bond Energy (kJ/mol) | Type of Process |
|---|---|---|
| H–H | 436 | Bond breaking (endothermic) |
| O=O | 498 | Bond breaking (endothermic) |
| O–H | 463 | Bond formation (exothermic) |
Steps for Calculating Energy Change Using Bond Energies
- Write the balanced chemical equation.
- List all the bonds broken (reactants) and bonds formed (products).
- Use the bond energy table to calculate total energy absorbed (to break bonds) and total energy released (to form bonds).
- Apply the formula: \( \mathrm{ΔH = Σ(Bonds\ Broken) – Σ(Bonds\ Formed)} \)
- Determine whether it is endothermic or exothermic based on the sign of \( \mathrm{ΔH} \).
Example
Explain whether the reaction \( \mathrm{H_2 + Cl_2 \rightarrow 2HCl} \) is endothermic or exothermic, given the following bond energies:
H–H = 436 kJ/mol, Cl–Cl = 243 kJ/mol, H–Cl = 431 kJ/mol.
▶️ Answer / Explanation
Step 1: Bonds broken: H–H and Cl–Cl = \( \mathrm{436 + 243 = 679\ kJ/mol} \)
Step 2: Bonds formed: 2 × H–Cl = \( \mathrm{2 \times 431 = 862\ kJ/mol} \)
Step 3: \( \mathrm{ΔH = 679 – 862 = -183\ kJ/mol} \)
Final Answer: \( \mathrm{ΔH < 0} \), so the reaction is exothermic.
Example
Calculate the enthalpy change for the reaction \( \mathrm{CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O} \) using the following bond energies:
- C–H = 412 kJ/mol
- O=O = 498 kJ/mol
- C=O = 743 kJ/mol
- O–H = 463 kJ/mol
▶️ Answer / Explanation
Step 1: Bonds broken = (4 × C–H) + (2 × O=O) = \( \mathrm{4(412) + 2(498) = 2644\ kJ/mol} \)
Step 2: Bonds formed = (2 × C=O) + (4 × O–H) = \( \mathrm{2(743) + 4(463) = 3338\ kJ/mol} \)
Step 3: \( \mathrm{ΔH = 2644 – 3338 = -694\ kJ/mol} \)
Final Answer: \( \mathrm{ΔH = -694\ kJ/mol} \), exothermic reaction (energy released).
Example
For the reaction \( \mathrm{N_2 + O_2 \rightarrow 2NO} \), calculate the enthalpy change using bond energies: N≡N = 945 kJ/mol, O=O = 498 kJ/mol, N=O = 607 kJ/mol.
▶️ Answer / Explanation
Step 1: Bonds broken: N≡N + O=O = \( \mathrm{945 + 498 = 1443\ kJ/mol} \)
Step 2: Bonds formed: 2 × N=O = \( \mathrm{2(607) = 1214\ kJ/mol} \)
Step 3: \( \mathrm{ΔH = 1443 – 1214 = +229\ kJ/mol} \)
Final Answer: \( \mathrm{ΔH = +229\ kJ/mol} \), an endothermic reaction (energy absorbed).
