IB MYP 4-5 Chemistry -Extraction of metals (electrolysis, carbon reduction)- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Extraction of metals (electrolysis, carbon reduction)- Study Notes
Key Concepts
- Extraction of Metals
Extraction of Metals
Extraction of Metals
The extraction of metals is the process of obtaining pure metals from their ores (naturally occurring compounds of metals) using chemical or electrochemical methods.
The method used to extract a metal depends on its position in the reactivity series. Highly reactive metals require more energy or advanced methods (like electrolysis), while less reactive metals can be extracted by simple chemical reduction.
Important Terms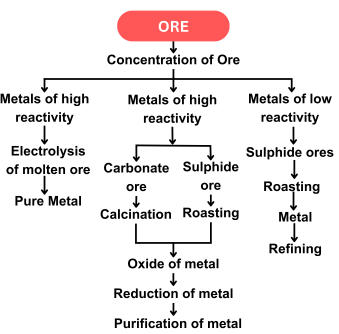
- Ore: A naturally occurring rock containing enough metal or metal compound to make extraction economically worthwhile.
- Gangue: The impurities such as sand, clay, and rock mixed with the ore.
- Metallurgy: The entire process of extracting metals from ores and refining them for use.
- Reduction: The process of converting metal compounds into free metal by removing oxygen.
Stages in the Extraction of Metals
- Concentration of Ore: Removing impurities (gangue) from the ore using physical methods like washing, froth flotation, or magnetic separation.
- Extraction of Metal: Converting metal compounds into free metals using chemical reduction or electrolysis.
- Refining of Metal: Purifying the crude metal obtained after extraction.
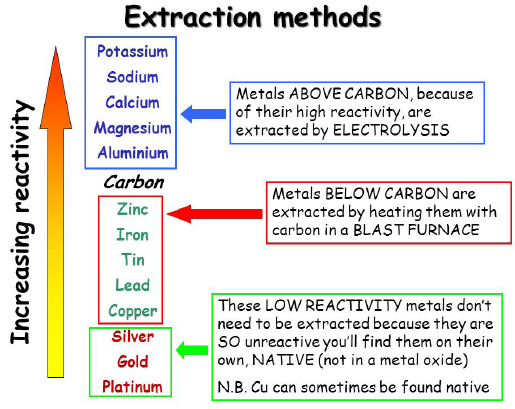
Methods of Metal Extraction Based on Reactivity
| Metal Reactivity | Examples | Extraction Method | Example Reaction |
|---|---|---|---|
| Very Reactive (top of series) | K, Na, Ca, Al | Electrolysis of molten salts | \( \mathrm{2Al_2O_3 \rightarrow 4Al + 3O_2} \) |
| Moderately Reactive | Zn, Fe, Pb | Reduction using carbon (or carbon monoxide) | \( \mathrm{Fe_2O_3 + 3CO \rightarrow 2Fe + 3CO_2} \) |
| Less Reactive (bottom of series) | Cu, Hg, Ag, Au | Thermal decomposition or found native | \( \mathrm{2HgO \rightarrow 2Hg + O_2} \) |
Extraction Examples by Reactivity
Each metal’s extraction method depends on how strongly it is bonded in its ore and how easily it can be reduced. Metals higher in the reactivity series form more stable compounds and need stronger or more energy-intensive methods (like electrolysis), while less reactive metals can be extracted by simple heating or chemical reduction.
(a) Extraction of Aluminium — by Electrolysis (High Reactivity)
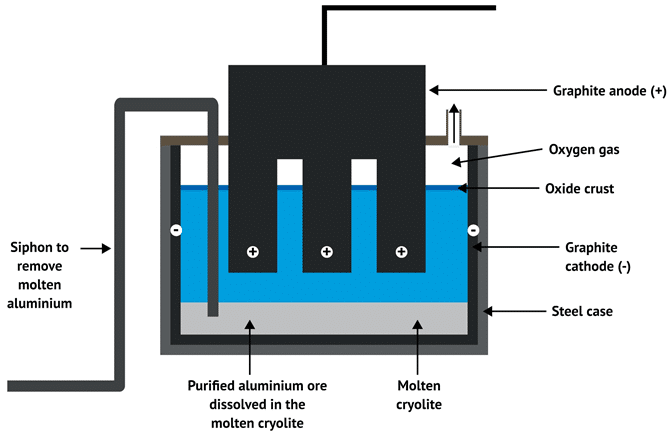
- Metal Reactivity: Aluminium is highly reactive and cannot be reduced by carbon.
- Ore: Bauxite (\( \mathrm{Al_2O_3 \cdot 2H_2O} \))
- Purification: Impurities are removed by the Bayer process to obtain pure alumina (\( \mathrm{Al_2O_3} \)).
- Process: Electrolysis of molten alumina mixed with cryolite (\( \mathrm{Na_3AlF_6} \)) to lower melting point and increase conductivity.
Electrolysis Cell Setup:
- Cathode: Carbon-lined steel container (where reduction occurs)
- Anode: Graphite rods (where oxidation occurs)
Reactions:
- At Cathode (Reduction): \( \mathrm{Al^{3+} + 3e^- \rightarrow Al} \)
- At Anode (Oxidation): \( \mathrm{2O^{2-} \rightarrow O_2 + 4e^-} \)
- Overall: \( \mathrm{2Al_2O_3 \rightarrow 4Al + 3O_2} \)
Additional Notes:
- Oxygen gas reacts with carbon anodes, forming \( \mathrm{CO_2} \), which means the anodes must be replaced periodically.
- The process is highly energy-intensive (requires electricity and high temperatures).
- Molten aluminium collects at the bottom and is tapped off periodically.
(b) Extraction of Iron — by Carbon Reduction (Medium Reactivity)
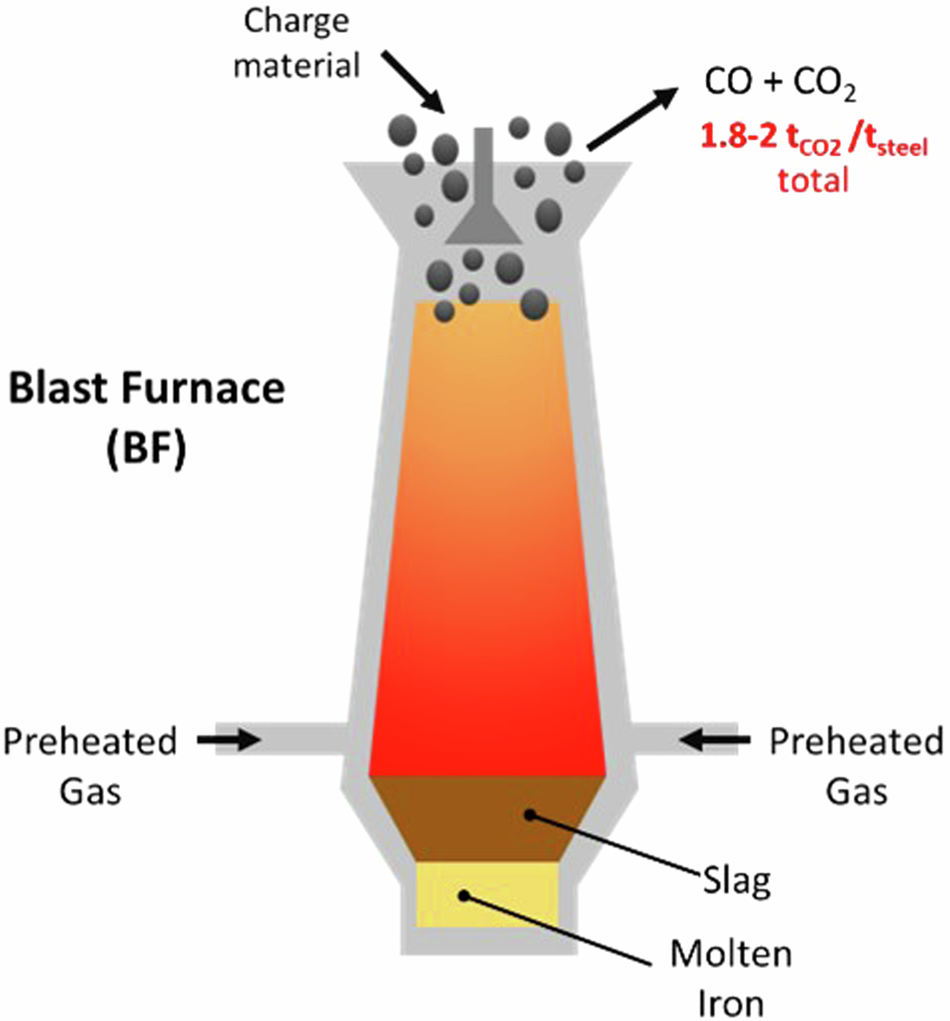
- Metal Reactivity: Iron is less reactive than aluminium and can be reduced using carbon (a cheaper reducing agent).
- Ore: Haematite (\( \mathrm{Fe_2O_3} \)) or Magnetite (\( \mathrm{Fe_3O_4} \))
- Furnace Used: Blast Furnace — a tall, heat-resistant steel tower lined with refractory bricks.
- Raw Materials:
- Iron ore (source of iron)
- Coke (carbon, acts as reducing agent)
- Limestone (\( \mathrm{CaCO_3} \)) — removes impurities (flux)
- Hot air — provides oxygen for combustion
Main Reactions in the Furnace:
- \( \mathrm{C + O_2 \rightarrow CO_2} \)
- \( \mathrm{CO_2 + C \rightarrow 2CO} \)
- \( \mathrm{Fe_2O_3 + 3CO \rightarrow 2Fe + 3CO_2} \)
Formation of Slag (Removal of Impurities):
- \( \mathrm{CaCO_3 \rightarrow CaO + CO_2} \)
- \( \mathrm{CaO + SiO_2 \rightarrow CaSiO_3 (slag)} \)
Additional Notes:
- Molten iron collects at the bottom of the furnace and is tapped off.
- Molten slag floats on top and is removed separately.
- The process is a redox reaction: iron(III) oxide is reduced and carbon monoxide is oxidized.
(c) Extraction of Copper — by Roasting and Reduction (Low Reactivity)
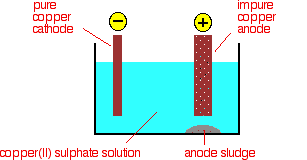
- Metal Reactivity: Copper is a moderately low reactive metal — its compounds can be reduced by heating.
- Ores: Copper(I) sulfide (\( \mathrm{Cu_2S} \)), Copper(II) carbonate (\( \mathrm{CuCO_3} \)), or Copper(II) oxide (\( \mathrm{CuO} \))
Step 1 — Roasting of Sulfide Ores:
- \( \mathrm{2Cu_2S + 3O_2 \rightarrow 2Cu_2O + 2SO_2} \)
Step 2 — Reduction of Oxide:
- \( \mathrm{Cu_2O + Cu_2S \rightarrow 6Cu + SO_2} \)
Alternative Method (Carbon Reduction):
- \( \mathrm{CuO + C \rightarrow Cu + CO} \)
Additional Notes:
- Some copper is purified further by electrolysis (electrorefining).
- The copper produced by roasting contains impurities like Fe, Ag, and Au.
- Blister copper (98–99% pure) is obtained before refining.
(d) Extraction of Zinc — by Roasting and Reduction
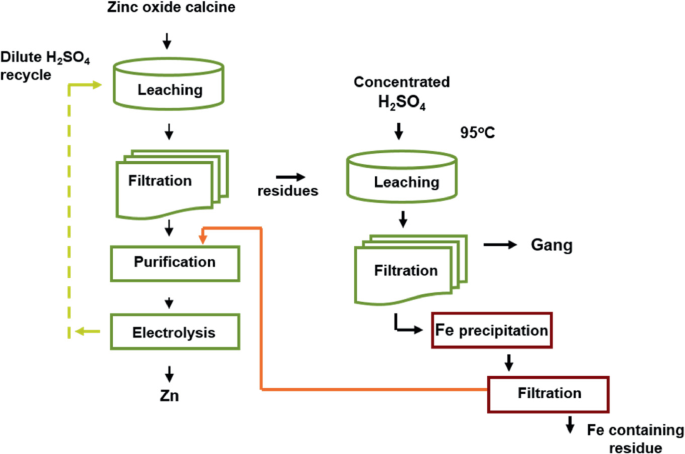
- Ore: Zinc blende (\( \mathrm{ZnS} \))
- Step 1: Roasting: \( \mathrm{2ZnS + 3O_2 \rightarrow 2ZnO + 2SO_2} \)
- Step 2: Reduction: \( \mathrm{ZnO + C \rightarrow Zn + CO} \)
- Metal zinc vaporizes and is condensed at cooler regions of the furnace.
Additional Notes:
- Zinc extraction requires careful temperature control to prevent reoxidation of zinc vapor.
- Final purification done by distillation or electrolysis.
(e) Extraction of Gold and Silver — Native or Chemical Reduction (Very Low Reactivity)
- Metal Reactivity: Gold and silver lie at the bottom of the reactivity series; they do not react with oxygen or water.
- Occurrence: Found native (in pure metallic form) in rocks or alluvial deposits.
- Extraction Methods:
- Mechanical separation (washing, panning)
- Chemical extraction using cyanide process (for silver and gold ores)
Cyanide Process (for Silver/Gold):
- \( \mathrm{4Au + 8NaCN + 2H_2O + O_2 \rightarrow 4Na[Au(CN)_2] + 4NaOH} \)
- \( \mathrm{2Na[Au(CN)_2] + Zn \rightarrow Na_2[Zn(CN)_4] + 2Au} \)
Additional Notes:
- This process is a redox displacement reaction — zinc, being more reactive, displaces gold or silver from solution.
- Gold and silver are purified by melting and refining after recovery.
Environmental and Economic Considerations
- Electrolysis consumes large amounts of electricity → costly.
- Carbon reduction produces \( \mathrm{CO_2} \) → contributes to greenhouse gases.
- Extraction methods must balance efficiency, cost, and environmental safety.
Example
Why can metals like gold and silver be found in their native (uncombined) state in nature?
▶️ Answer / Explanation
Step 1: Gold and silver are very low in the reactivity series.
Step 2: They do not react easily with oxygen, water, or other substances.
Final Answer: They exist in their pure metallic form because they are chemically very unreactive.
Example
Why is cryolite (\( \mathrm{Na_3AlF_6} \)) used in the extraction of aluminium by electrolysis?
▶️ Answer / Explanation
Step 1: Pure aluminium oxide has a very high melting point (~2050°C).
Step 2: Cryolite lowers the melting point and increases electrical conductivity.
Final Answer: Cryolite reduces energy cost and allows smooth electrolysis at ~950°C.
Example
Iron is extracted in a blast furnace using carbon monoxide as a reducing agent. Explain the redox reactions that occur inside the furnace.
▶️ Answer / Explanation
Step 1: Formation of reducing agent:
\( \mathrm{C + O_2 \rightarrow CO_2} \)
\( \mathrm{CO_2 + C \rightarrow 2CO} \)
Step 2: Reduction of iron oxide:
\( \mathrm{Fe_2O_3 + 3CO \rightarrow 2Fe + 3CO_2} \)
Step 3: Identify redox behavior:
- \( \mathrm{Fe_2O_3} \) is reduced to \( \mathrm{Fe} \) (gain of electrons).
- \( \mathrm{CO} \) is oxidized to \( \mathrm{CO_2} \) (loss of electrons).
Final Answer: The blast furnace works on redox principles — carbon monoxide reduces iron oxide while itself being oxidized.
