IB MYP 4-5 Chemistry -Factors affecting rate — temperature, concentration, surface area, catalysts- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Factors affecting rate — temperature, concentration, surface area, catalysts- Study Notes
Key Concepts
- Factors Affecting the Rate of Reaction — Temperature, Concentration, Surface Area, and Catalysts
Factors Affecting the Rate of Reaction — Temperature, Concentration, Surface Area, and Catalysts
Factors Affecting the Rate of Reaction — Temperature, Concentration, Surface Area, and Catalysts
The rate of a chemical reaction measures how fast reactants are converted into products. The rate depends on how frequently and effectively particles collide, as explained by the collision theory.
\( \mathrm{Rate\ of\ Reaction = \dfrac{Change\ in\ Concentration}{Change\ in\ Time}} \)
![]()
Several factors influence the frequency and energy of collisions, thereby affecting the reaction rate.
Temperature
Effect: Increasing temperature increases the rate of reaction.
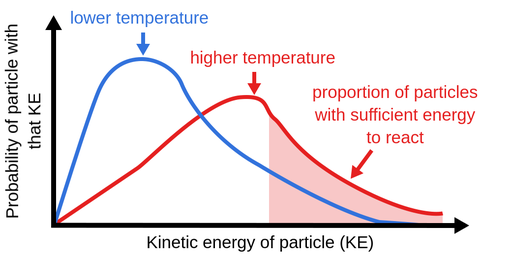
- At higher temperatures, particles move faster and have higher kinetic energy.
- More collisions per second → more chance of successful collisions.
- A greater fraction of particles have energy ≥ activation energy \( (\mathrm{E_a}) \).
Result: Both collision frequency and collision energy increase → faster reaction.
Example: Magnesium reacts more rapidly with hot hydrochloric acid than with cold acid.
Concentration (or Pressure for Gases)
Effect: Increasing concentration (or pressure for gases) increases the rate of reaction.

- Higher concentration → more particles per unit volume.
- In gases, higher pressure brings particles closer together.
- This increases the frequency of collisions between reactant particles.
Result: More effective collisions → higher rate of reaction.
Example: A concentrated acid reacts faster with metals than a dilute acid.
Surface Area
Effect: Increasing surface area increases the rate of reaction.
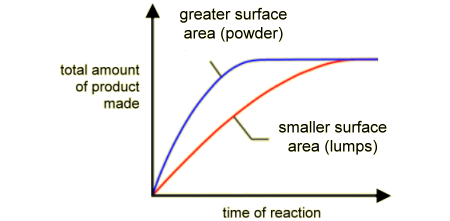
- Smaller particle size or powder form → larger total surface area for collisions.
- More exposed particles → more frequent and effective collisions.

Result: Faster reaction because more reactant particles are available for contact.
Example: Powdered calcium carbonate reacts faster with acid than marble chips.
Catalyst
Effect: A catalyst increases the rate of a reaction without being consumed
 .
.
- Provides an alternative reaction pathway with a lower activation energy.
- More particles have energy ≥ \( \mathrm{E_a} \), leading to more effective collisions.
- Does not affect the overall energy change \( (\mathrm{ΔH}) \).
Example: Manganese dioxide (\( \mathrm{MnO_2} \)) speeds up the decomposition of hydrogen peroxide.
Factors Affecting Rate of Reaction
| Factor | How It Changes Collisions | Effect on Rate | Example |
|---|---|---|---|
| Temperature | Increases kinetic energy → more frequent and energetic collisions | Rate increases | Mg reacts faster in hot acid |
| Concentration / Pressure | More particles per volume → more collisions | Rate increases | Concentrated acid reacts faster |
| Surface Area | More particles exposed → higher collision frequency | Rate increases | Powdered CaCO₃ reacts faster than chips |
| Catalyst | Lowers activation energy → more effective collisions | Rate increases | MnO₂ catalyzes H₂O₂ decomposition |
Example
Hydrochloric acid reacts with magnesium ribbon. When the acid is heated, the reaction is faster. Explain why.
▶️ Answer / Explanation
Step 1: Higher temperature increases kinetic energy of particles.
Step 2: More collisions occur per second and more particles have energy ≥ \( \mathrm{E_a} \).
Final Answer: The reaction is faster because there are more effective collisions at higher temperature.
Example
Calcium carbonate reacts with hydrochloric acid. When powdered CaCO₃ is used instead of chips, the reaction speeds up. Explain this observation.
▶️ Answer / Explanation
Step 1: Powdered calcium carbonate has a greater surface area.
Step 2: More acid particles can collide with the solid at the same time.
Step 3: More frequent collisions → faster reaction.
Final Answer: Increasing surface area increases collision frequency, speeding up the reaction.
Example
In the decomposition of hydrogen peroxide, the rate doubles when temperature increases from 25°C to 35°C. Explain using collision theory and activation energy.
▶️ Answer / Explanation
Step 1: At higher temperature, H₂O₂ molecules move faster and collide more often.
Step 2: A greater fraction of molecules have kinetic energy ≥ \( \mathrm{E_a} \).
Step 3: Therefore, more collisions are effective and reaction rate increases.
Final Answer: Temperature increases the proportion of molecules with enough energy to overcome \( \mathrm{E_a} \), making the reaction proceed faster.
