IB MYP 4-5 Chemistry -Filtration, evaporation, and crystallization- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Filtration, evaporation, and crystallization- Study Notes
Key Concepts
- Separation Techniques for Mixtures: Filtration, Evaporation, and Crystallization
Separation Techniques for Mixtures: Filtration, Evaporation, and Crystallization
Separation Techniques for Mixtures: Filtration, Evaporation, and Crystallization
Different components of a mixture can be separated based on differences in their physical properties such as particle size, solubility, and boiling point. Three important physical methods are filtration, evaporation, and crystallization.
Filtration 
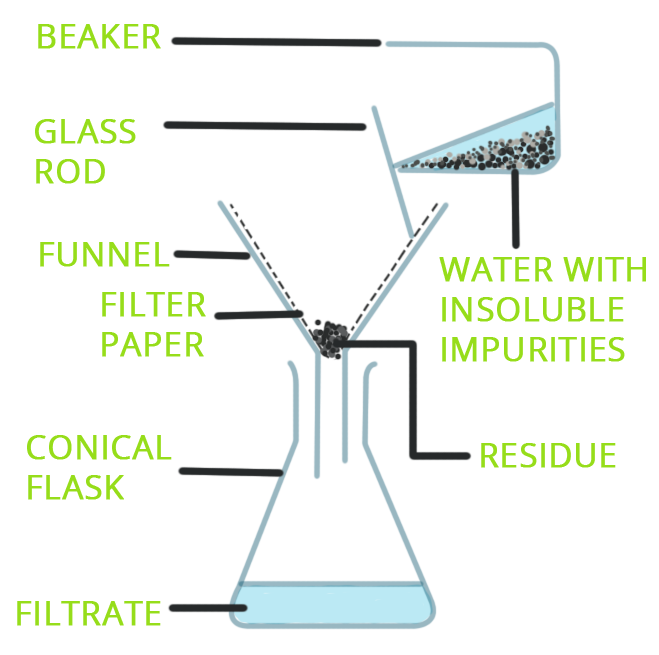
Filtration is a method used to separate an insoluble solid from a liquid or a solution.
- The mixture is poured through a filter paper placed in a funnel.
- The liquid that passes through is called the filtrate.
- The solid that remains on the paper is called the residue.
Principle: It works because the solid particles are too large to pass through the tiny pores of the filter paper, while the liquid particles are small enough to pass through.
Examples:
- Separating sand from a mixture of sand and water
- Separating chalk powder from water
- Removing coffee grounds from brewed coffee
| Component | Description |
|---|---|
| Residue | Solid particles left on the filter paper |
| Filtrate | Clear liquid that passes through the filter paper |
Evaporation
Evaporation is used to separate a soluble solid from a liquid by heating the solution until the solvent evaporates, leaving the solid behind.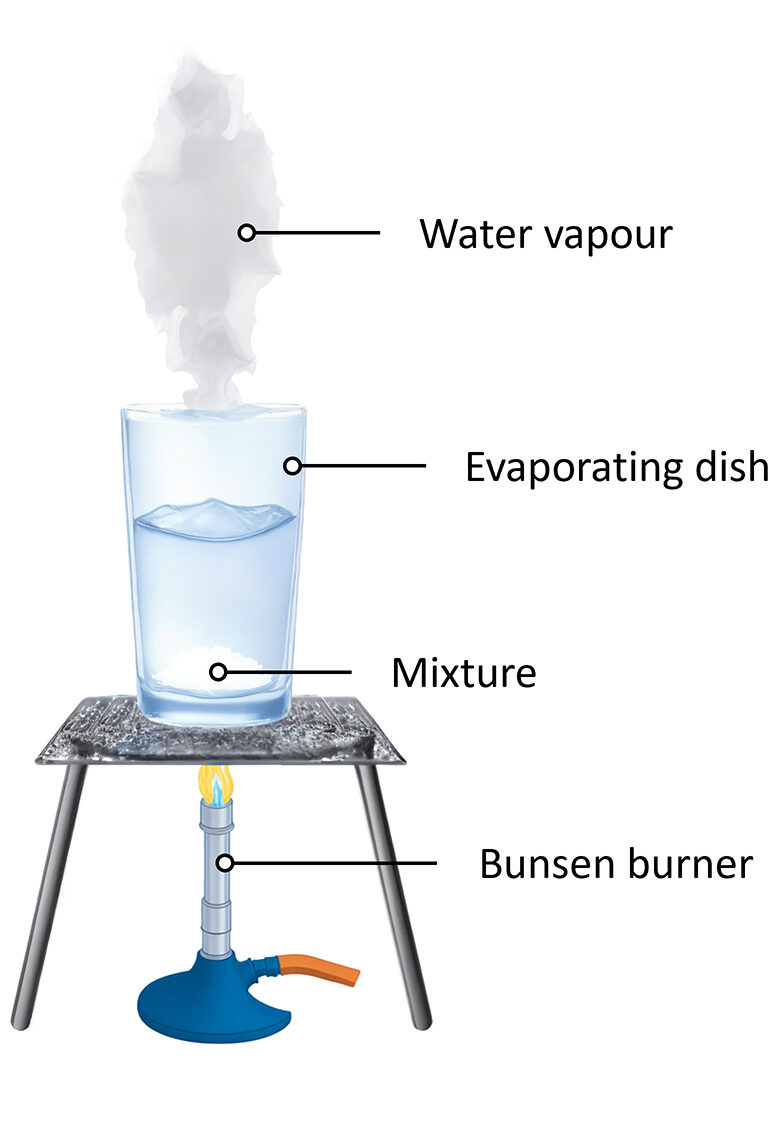
- The solvent changes to vapor (gas) and escapes into the air.
- The solute remains as a solid residue.
- The method is useful when recovery of the solvent is not needed.
Principle: The substance with the lower boiling point (usually the solvent) evaporates first, while the solid with a higher boiling point remains behind.
Examples:
- Obtaining salt from seawater
- Concentrating sugar syrup
- Drying clothes (water evaporates, cloth remains)
Limitations:
- Some solids may decompose on strong heating.
- Solvent cannot be recovered in this method.
Crystallization
Crystallization is a method used to obtain pure solid crystals of a substance from its solution.
- It separates a dissolved solid (solute) from a solution when both solute and solvent are desired in pure form.
- The solution is heated to evaporate part of the solvent, making it concentrated.
- Then it is cooled, and the solute forms crystals as it becomes less soluble at lower temperatures.
Principle: Substances have different solubilities at different temperatures — solubility decreases as temperature falls, causing the solute to crystallize out.
Steps in Crystallization:
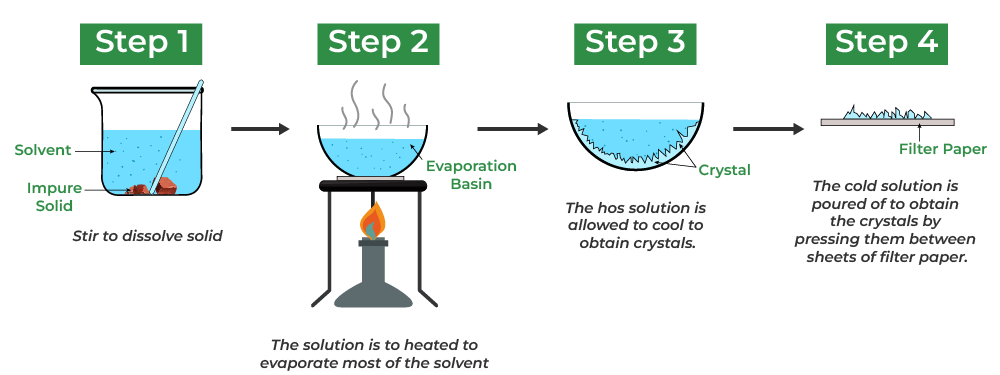
- Prepare a saturated solution by heating the solute in a solvent.
- Filter to remove any insoluble impurities.
- Cool the hot solution slowly to form crystals.
- Separate and dry the crystals using filter paper.
Examples:
- Obtaining pure copper sulfate crystals from its solution
- Purifying sugar or salt from impure samples
- Making crystals in laboratory experiments
Comparison Table: Filtration, Evaporation, and Crystallization
| Method | Purpose | Type of Mixture Separated | Example |
|---|---|---|---|
| Filtration | To separate an insoluble solid from a liquid | Solid + Liquid (heterogeneous) | Sand + Water |
| Evaporation | To recover the solute by evaporating the solvent | Solid dissolved in Liquid (homogeneous) | Salt + Water |
| Crystallization | To obtain pure crystals of solute from a solution | Solid dissolved in Liquid (homogeneous) | Copper sulfate + Water |
Key Differences
- Filtration removes insoluble impurities.
- Evaporation recovers solute but not solvent.
- Crystallization gives pure, solid crystals and retains both solute and solvent purity.
Scientific Principle Behind Each
- Filtration: Based on difference in particle size.
- Evaporation: Based on difference in boiling point.
- Crystallization: Based on difference in solubility at different temperatures.
Example:
How can a mixture of sand and salt be separated completely using filtration and evaporation?
▶️ Answer / Explanation
Step 1: Add water to dissolve the salt; sand remains undissolved.
Step 2: Filter the mixture — sand (residue) remains on the filter paper, and salt solution (filtrate) passes through.
Step 3: Evaporate the filtrate to remove water, leaving pure salt crystals.
Final Answer: The mixture is separated by filtration (removing sand) and evaporation (recovering salt).
Example :
A student heats 100 g of a salt solution and obtains 25 g of dry salt. Calculate the mass of water evaporated.
▶️ Answer / Explanation
Step 1: Total mass of solution = 100 g.
Step 2: Mass of salt obtained = 25 g.
Step 3: Water evaporated = 100 − 25 = 75 g.
Final Answer: 75 g of water evaporated during heating.
Example :
Why is crystallization preferred over evaporation to obtain pure crystals of a solute from a solution?
▶️ Answer / Explanation
Step 1: In evaporation, impurities that do not dissolve remain mixed with the solute.
Step 2: Some solids may decompose or lose water of crystallization on strong heating.
Step 3: Crystallization allows slow cooling so that only pure solute crystallizes out, leaving impurities in solution.
Final Answer: Crystallization is preferred because it produces pure, well-formed crystals without decomposing or mixing impurities.
