IB MYP 4-5 Chemistry -Formation of ions and ionic lattices- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Formation of ions and ionic lattices- Study Notes
Key Concepts
- Formation of Ions and Ionic Lattices
Formation of Ions and Ionic Lattices
Formation of Ions and Ionic Lattices
When atoms lose or gain electrons to achieve a stable outer electron configuration (usually a full valence shell), they form ions. The electrostatic attraction between oppositely charged ions results in the formation of ionic compounds arranged in a repeating ionic lattice structure.
Formation of Ions![]()
An ion is a charged particle formed when an atom loses or gains electrons.
- Cation: A positively charged ion formed by losing electrons.
- Anion: A negatively charged ion formed by gaining electrons.
Why Atoms Form Ions:
- To achieve a stable electron configuration (like noble gases).
- To minimize potential energy and become more stable.
Examples of Ion Formation:
| Atom | Electron Change | Ion Formed | Type of Ion |
|---|---|---|---|
| \( \mathrm{Na} \) (2,8,1) | Loses 1 e⁻ | \( \mathrm{Na^+} \) | Cation |
| \( \mathrm{Cl} \) (2,8,7) | Gains 1 e⁻ | \( \mathrm{Cl^-} \) | Anion |
| \( \mathrm{Mg} \) (2,8,2) | Loses 2 e⁻ | \( \mathrm{Mg^{2+}} \) | Cation |
Equation Example:
\( \mathrm{Na \rightarrow Na^+ + e^-} \)
\( \mathrm{Cl + e^- \rightarrow Cl^-} \)
\( \mathrm{Na^+ + Cl^- \rightarrow NaCl} \)
Formation of Ionic Compounds![]()
Ionic compounds form through electrostatic attraction between positive and negative ions. This attraction is known as an ionic bond.
![]()
Steps in Ionic Compound Formation:
- Metal atom loses electrons → forms cation.
- Non-metal atom gains those electrons → forms anion.
- Oppositely charged ions attract → form neutral compound.
Example – Formation of Magnesium Chloride (\( \mathrm{MgCl_2} \)):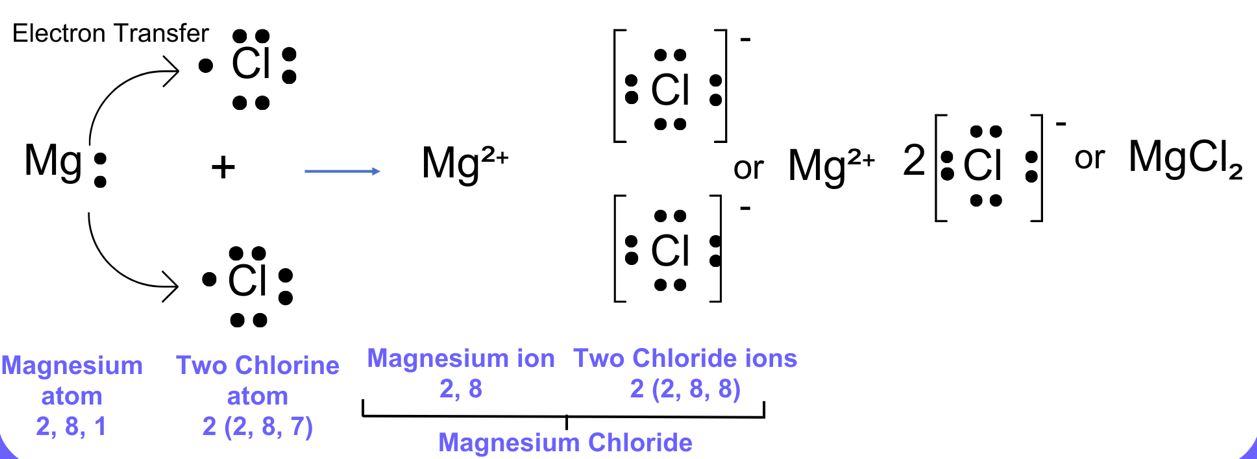
- \( \mathrm{Mg} \) loses two electrons → \( \mathrm{Mg^{2+}} \).
- Each \( \mathrm{Cl} \) gains one electron → \( \mathrm{Cl^-} \).
- Two chloride ions are needed to balance charge: \( \mathrm{Mg^{2+} + 2Cl^- \rightarrow MgCl_2} \).
Properties of Ionic Compounds:
- High melting and boiling points (strong ionic bonds).
- Solid at room temperature.
- Conduct electricity when molten or dissolved (ions move freely).
- Usually soluble in water.
- Form crystalline solids.
Ionic Lattice Structure
An ionic lattice is a giant 3D structure formed by the regular repeating arrangement of positive and negative ions held together by strong electrostatic forces.
![]()
Key Features:
- Each ion is surrounded by oppositely charged ions in a fixed pattern.
- Forces act in all directions — giving ionic solids strength and stability.
- Example: In sodium chloride, each \( \mathrm{Na^+} \) is surrounded by 6 \( \mathrm{Cl^-} \) ions and vice versa (6:6 coordination).
Diagram Description (MYP-level): Imagine a cube-like 3D arrangement where \( \mathrm{Na^+} \) and \( \mathrm{Cl^-} \) alternate in all directions. The repeating pattern forms a crystal lattice — strong, symmetrical, and stable.
Properties Due to Ionic Lattice:![]()
- High melting/boiling points: Large energy needed to overcome strong electrostatic attractions.
- Brittle: When like charges align during stress, they repel and the lattice breaks.
- Electrical conductivity: Only when ions are free to move (molten/solution state).
Formation and Properties of Ionic Compounds
| Feature | Description | Reason/Explanation |
|---|---|---|
| Bond Type | Ionic (electrostatic attraction) | Between oppositely charged ions |
| Structure | Giant 3D lattice | Repeating ion arrangement with strong forces |
| Melting Point | High | Strong ionic bonds require much energy to break |
| Conductivity | Only when molten or aqueous | Ions free to move and carry charge |
| Brittleness | Brittle under force | Like charges align and repel when lattice shifts |
Example:
Write the electronic structures of sodium and chlorine, and show how they form ions in sodium chloride.
▶️ Answer / Explanation
Sodium: 2,8,1 → loses 1 electron → \( \mathrm{Na^+} \) (2,8).
Chlorine: 2,8,7 → gains 1 electron → \( \mathrm{Cl^-} \) (2,8,8).
Result: \( \mathrm{Na^+Cl^-} \) held by electrostatic attraction → \( \mathrm{NaCl} \).
Example :
Explain why magnesium oxide (\( \mathrm{MgO} \)) has a higher melting point than sodium chloride (\( \mathrm{NaCl} \)).
▶️ Answer / Explanation
Step 1: \( \mathrm{MgO} \) has ions with charges \( \mathrm{Mg^{2+}} \) and \( \mathrm{O^{2-}} \).
Step 2: \( \mathrm{NaCl} \) has ions with charges \( \mathrm{Na^+} \) and \( \mathrm{Cl^-}} \).
Step 3: Stronger electrostatic forces exist between ions with higher charges.
Final Answer: \( \mathrm{MgO} \) has a higher melting point because its ionic bonds are stronger than those in \( \mathrm{NaCl} \).
Example :
Explain why ionic compounds conduct electricity when molten but not when solid, using the concept of the ionic lattice.
▶️ Answer / Explanation
Step 1: In solid state, ions are fixed in a lattice → cannot move.
Step 2: When molten, the lattice breaks and ions become mobile.
Step 3: Moving ions carry electric current.
Final Answer: Ionic solids do not conduct because ions are immobile; conductivity occurs when molten or dissolved due to free ion movement.
