IB MYP 4-5 Chemistry -Fractional distillation of crude oil- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Fractional distillation of crude oil- Study Notes
Key Concepts
- Fractional Distillation of Crude Oil
Fractional Distillation of Crude Oil
Fractional Distillation of Crude Oil
Crude oil (also known as petroleum) is a complex mixture of hydrocarbons — compounds made only of carbon (C) and hydrogen (H). It is a non-renewable fossil fuel formed from the remains of ancient marine organisms buried under heat and pressure over millions of years.
Because crude oil contains hydrocarbons with different chain lengths and boiling points, it must be separated by fractional distillation before use.
![]()
Principle of Fractional Distillation
- Fractional distillation separates a mixture into parts (fractions) based on their different boiling points.
- Hydrocarbons with shorter chains have lower boiling points and rise higher in the column.
- Hydrocarbons with longer chains have higher boiling points and condense lower in the column.
Key Idea: Each fraction contains hydrocarbons with similar boiling ranges and properties.
Steps in Fractional Distillation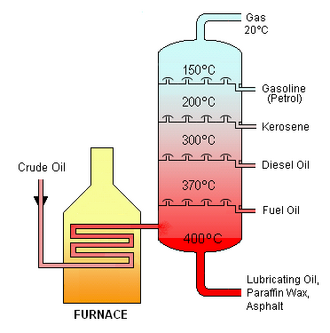
- Heating: Crude oil is heated in a furnace until it vaporizes (about 400°C).
- Entering the column: The vapor enters the bottom of a tall fractionating column.
- Temperature gradient: The column is hot at the bottom and cooler at the top.
- Condensation: Hydrocarbon vapors rise and condense at different heights according to their boiling points.
- Collection: Fractions are collected on trays at different levels of the column.
Diagram Description of the Fractionating Column
- Bottom: Hottest region (~400°C) → heavy fractions like bitumen condense.
- Middle: Medium-temperature regions → kerosene and diesel condense.
- Top: Coolest region (~20°C) → light fractions like gasoline and refinery gases escape.
Major Fractions of Crude Oil and Their Uses
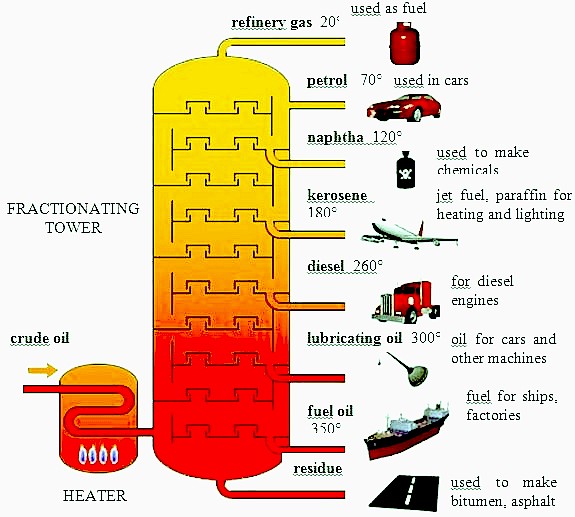
| Fraction | Boiling Range (°C) | Hydrocarbon Size | Main Uses |
|---|---|---|---|
| Refinery gases | Below 40°C | 1–4 carbon atoms | Cooking fuel (LPG), heating |
| Petrol (Gasoline) | 40–100°C | 5–10 carbon atoms | Fuel for cars |
| Naphtha | 100–180°C | 6–11 carbon atoms | Feedstock for chemicals and plastics |
| Kerosene | 180–250°C | 10–16 carbon atoms | Jet fuel, lamps, heaters |
| Diesel oil | 250–350°C | 15–20 carbon atoms | Fuel for trucks, buses |
| Lubricating oil / Fuel oil | 350–500°C | 20–30 carbon atoms | Lubricants, ship fuel |
| Bitumen | Above 500°C | >30 carbon atoms | Road surfacing, roofing |
Trends in Physical Properties of Fractions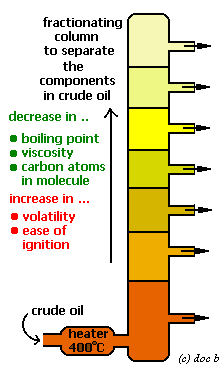
- As the chain length increases:
- Boiling point increases
- Viscosity increases (thicker)
- Flammability decreases
- Color becomes darker
Importance of Fractional Distillation
- Provides fuels for transport and industry (petrol, diesel, kerosene).
- Supplies raw materials (naphtha) for producing plastics, detergents, and solvents.
- Ensures efficient use of crude oil through separation and refinement.
Example
Which fraction of crude oil is collected at the top of the fractionating column, and why?
▶️ Answer / Explanation
Step 1: The top of the column is the coolest region.
Step 2: Only hydrocarbons with very low boiling points remain as gases.
Step 3: Refinery gases (e.g., methane, ethane, propane, butane) are collected at the top.
Final Answer: Refinery gases are collected at the top because they have the lowest boiling points.
Example
Explain why diesel oil is more viscous and less flammable than petrol.
▶️ Answer / Explanation
Step 1: Diesel contains larger hydrocarbon molecules (longer carbon chains) than petrol.
Step 2: Larger molecules have stronger intermolecular forces.
Step 3: Therefore, diesel has a higher boiling point, is thicker (more viscous), and burns less easily.
Final Answer: Diesel is less flammable and more viscous because its hydrocarbons are larger and have stronger forces between molecules.
Example
Crude oil is heated to 400°C and fed into a fractional distillation column. Explain why heavy fractions like bitumen remain at the bottom while lighter fractions like petrol rise to the top.
▶️ Answer / Explanation
Step 1: Each hydrocarbon fraction has a different boiling point depending on its chain length.
Step 2: Bitumen has very long chains with very high boiling points, so it does not vaporize completely and remains as a liquid at the bottom.
Step 3: Petrol has shorter chains and lower boiling points, so it vaporizes easily and rises to condense at cooler upper parts of the column.
Final Answer: Separation occurs because lighter fractions vaporize and rise, while heavier ones remain liquid at the bottom due to higher boiling points.
