IB MYP 4-5 Chemistry -Half-life and decay curves- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Half-life and decay curves- Study Notes
Key Concepts
- Half-Life and Decay Curves
- Radioactive Decay Calculations
Half-Life and Decay Curves
Half-Life and Decay Curves
The half-life of a radioactive isotope is the time required for half of the radioactive nuclei in a sample to decay into more stable nuclei.
It represents the rate of radioactive decay a measure of how quickly or slowly a radioactive substance loses its activity.
Symbol: \( \mathrm{t_{1/2}} \)
![]()
Concept of Half-Life
- Each radioactive isotope has a constant half-life, unaffected by temperature, pressure, or chemical conditions.
- After each half-life, the amount of radioactive atoms decreases by half.
- As atoms decay, the activity (count rate) of the sample also halves.
Example Concept:
- Start with 100 g of a radioactive isotope.
- After 1 half-life → 50 g remains.
- After 2 half-lives → 25 g remains.
- After 3 half-lives → 12.5 g remains.
Formula for Radioactive Decay:
\( \mathrm{N = N_0 \left( \dfrac{1}{2} \right)^{t / t_{1/2}}} \)
- \( \mathrm{N_0} \): initial amount of substance
- \( \mathrm{N} \): remaining amount after time \( \mathrm{t} \)
- \( \mathrm{t_{1/2}} \): half-life of the isotope
Characteristics of Radioactive Decay
- Radioactive decay is a random process we cannot predict which atom will decay next.
- However, the overall rate follows a predictable exponential pattern.
- The number of undecayed nuclei or the activity of a sample decreases exponentially over time.
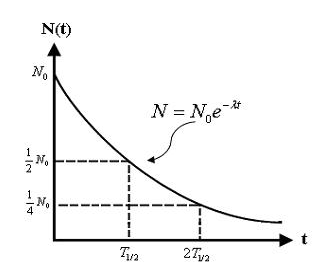
Mathematical Form of Exponential Decay:
\( \mathrm{N = N_{0} e^{-\lambda t}} \)
- \( \mathrm{\lambda} \): decay constant, related to half-life by \( \mathrm{\lambda = \dfrac{\ln(2)}{t_{1/2}}} \)
Graphical Representation — Decay Curves
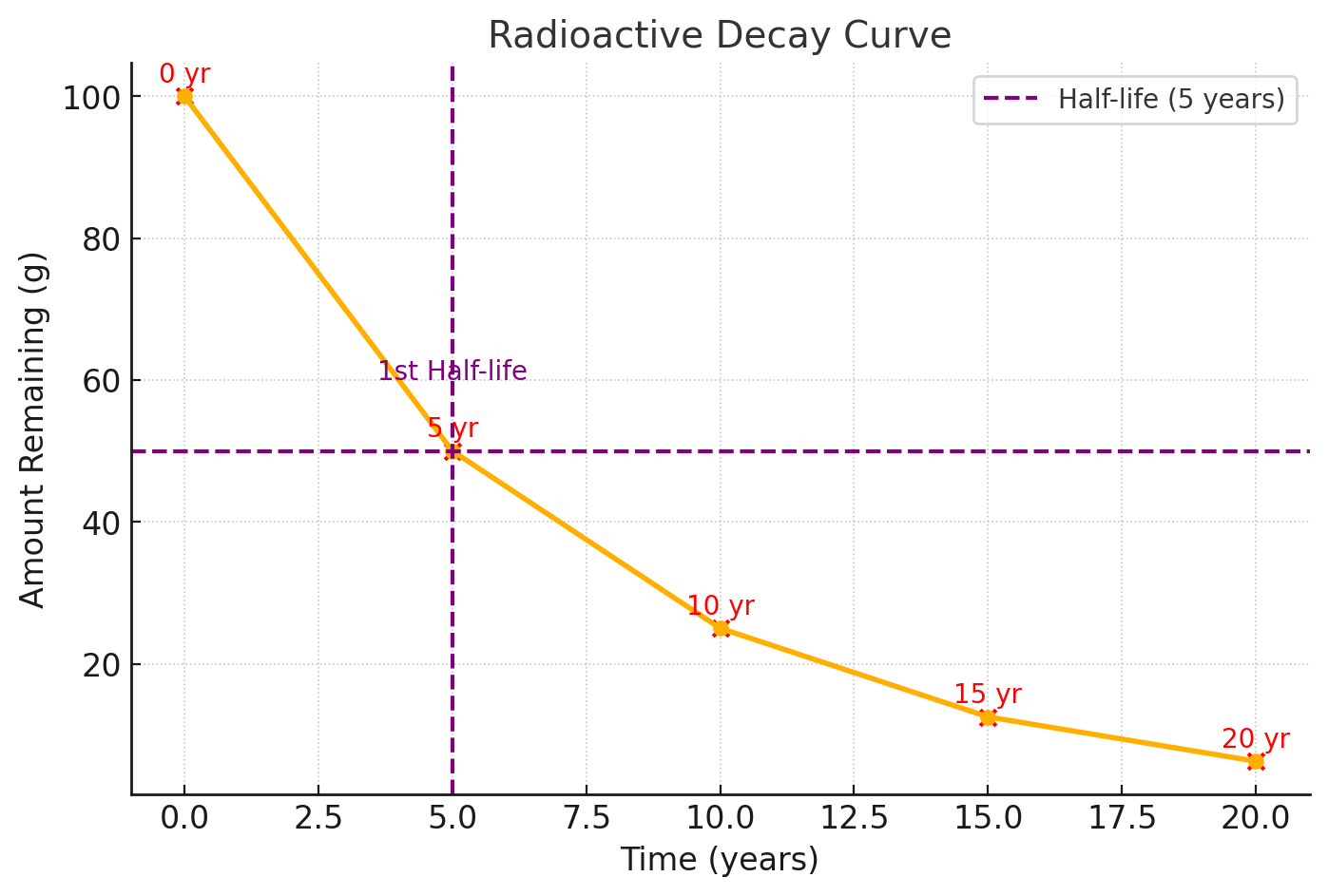
A decay curve shows how the quantity of a radioactive isotope decreases with time.
Features of a Decay Curve:
- The curve is always downward sloping (exponential decay).
- It never reaches zero — there is always some radioactive material left.
- Each half-life interval corresponds to a halving of the remaining atoms or activity.
Example: If 100 g of a substance has a half-life of 5 years, the decay curve will show:
| Time (years) | Amount Remaining (g) |
|---|---|
| 0 | 100 |
| 5 | 50 |
| 10 | 25 |
| 15 | 12.5 |
| 20 | 6.25 |
Shape of a Decay Curve:
- Starts at \( \mathrm{N_0} \) (initial activity or mass).

- Decreases rapidly at first, then more slowly as fewer atoms remain to decay.
- Approaches zero but never becomes exactly zero.
Determining Half-Life from a Decay Curve
- Plot a graph of number of undecayed nuclei (N) or activity vs. time (t).
- Find two points where activity is halved (e.g., from 100 → 50 → 25, etc.).
- The time interval between these points = one half-life.
Real-Life Applications of Half-Life
- Radiocarbon dating: Uses \( \mathrm{^{14}C} \) to date ancient materials.
- Medical tracers: Short half-life isotopes (e.g., \( \mathrm{^{99m}Tc} \)) diagnose body functions safely.
- Industrial uses: Measuring thickness, detecting leaks, and sterilizing medical equipment.
- Nuclear waste management: Understanding half-lives helps plan safe disposal of radioactive waste.
Half-Life Overview
| Quantity | Symbol | Meaning | Relationship |
|---|---|---|---|
| Half-life | \( \mathrm{t_{1/2}} \) | Time for half the nuclei to decay | \( \mathrm{t_{1/2} = \dfrac{\ln 2}{\lambda}} \) |
| Decay constant | \( \mathrm{\lambda} \) | Probability of decay per unit time | \( \mathrm{\lambda = \dfrac{\ln(2)}{t_{1/2}}} \) |
| Remaining amount | \( \mathrm{N} \) | Atoms remaining after time \( \mathrm{t} \) | \( \mathrm{N = N_0 (1/2)^{t/t_{1/2}}} \) |
Example
A radioactive isotope has a half-life of 10 years. If you start with 80 g, how much remains after 30 years?
▶️ Answer / Explanation
Step 1: \( \mathrm{t = 30\ years,\ t_{1/2} = 10\ years} \)
Step 2: Number of half-lives = \( \mathrm{30 / 10 = 3} \)
Step 3: \( \mathrm{N = 80 (1/2)^3 = 80/8 = 10\ g} \)
Final Answer: 10 g of the isotope remains after 30 years.
Example
The half-life of iodine-131 is 8 days. How long will it take for its activity to fall to one-eighth of its original value?
▶️ Answer / Explanation
Step 1: One-eighth = \( \mathrm{(1/2)^3} \) → three half-lives.
Step 2: Total time = \( \mathrm{3 \times 8 = 24\ days} \)
Final Answer: The activity falls to one-eighth in 24 days.
Example
A sample of \( \mathrm{^{60}Co} \) has a half-life of 5.3 years. If its initial activity is 640 counts per minute, what will be its activity after 15.9 years?
▶️ Answer / Explanation
Step 1: \( \mathrm{t = 15.9\ years,\ t_{1/2} = 5.3\ years} \)
Step 2: Number of half-lives = \( \mathrm{15.9 / 5.3 = 3} \)
Step 3: \( \mathrm{A = 640 (1/2)^3 = 640/8 = 80\ counts/min} \)
Final Answer: The activity after 15.9 years = 80 counts per minute.
Radioactive Decay Calculations
Radioactive Decay Calculations
Radioactive decay is the spontaneous transformation of an unstable nucleus into a more stable one, accompanied by the emission of radiation (α, β, or γ). The rate at which radioactive nuclei decay follows an exponential law and can be described mathematically using the decay constant and half-life.
Radioactive Decay Law
The number of undecayed nuclei at any time \( \mathrm{t} \) is given by:
\( \mathrm{N = N_0 e^{-\lambda t}} \)
- \( \mathrm{N_0} \): initial number of nuclei (or initial activity/mass)
- \( \mathrm{N} \): number of nuclei (or activity/mass) remaining after time \( \mathrm{t} \)
- \( \mathrm{\lambda} \): decay constant (probability of decay per unit time)
- \( \mathrm{t} \): time elapsed
Relationship Between Half-Life and Decay Constant:
\( \mathrm{t_{1/2} = \dfrac{\ln(2)}{\lambda} \quad \text{or} \quad \lambda = \dfrac{0.693}{t_{1/2}}} \)
Simplified Decay Formula Using Half-Life
When time \( \mathrm{t} \) is expressed in multiples of the half-life, the decay formula becomes:
\( \mathrm{N = N_0 \left(\dfrac{1}{2}\right)^{t/t_{1/2}}} \)
- Used to find the remaining quantity after a known time.
- Applicable to mass, number of atoms, or activity (they all decay at the same rate).
Decay Constant and Activity
The activity (rate of decay) of a radioactive sample is proportional to the number of undecayed nuclei.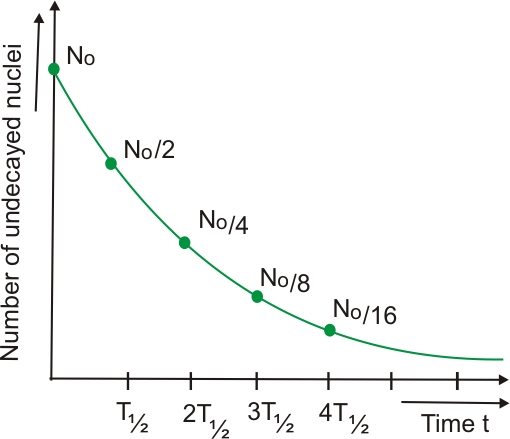
\( \mathrm{A = \lambda N} \)
- \( \mathrm{A} \): activity (decays per second, measured in becquerels — Bq)
- \( \mathrm{\lambda} \): decay constant
- \( \mathrm{N} \): number of radioactive atoms remaining
At any time \( \mathrm{t} \):
\( \mathrm{A = A_0 e^{-\lambda t}} \)
- \( \mathrm{A_0} \): initial activity
Common Types of Radioactive Decay Problems
| Type of Problem | Quantity to Find | Formula Used |
|---|---|---|
| Remaining mass or activity | \( \mathrm{N} \) | \( \mathrm{N = N_0 (1/2)^{t/t_{1/2}}} \) |
| Decay constant | \( \mathrm{\lambda} \) | \( \mathrm{\lambda = \dfrac{0.693}{t_{1/2}}} \) |
| Elapsed time | \( \mathrm{t} \) | \( \mathrm{t = t_{1/2} \dfrac{\log(N_0/N)}{\log(2)}} \) |
Important Relationships Summary
| Quantity | Symbol | Equation | Unit |
|---|---|---|---|
| Half-life | \( \mathrm{t_{1/2}} \) | \( \mathrm{t_{1/2} = \dfrac{0.693}{\lambda}} \) | seconds, minutes, years |
| Decay constant | \( \mathrm{\lambda} \) | \( \mathrm{\lambda = \dfrac{0.693}{t_{1/2}}} \) | s⁻¹ or yr⁻¹ |
| Remaining mass/activity | \( \mathrm{N} \) | \( \mathrm{N = N_0 (1/2)^{t/t_{1/2}}} \) | g or Bq |
Example
A radioactive isotope has a half-life of 6 hours. If 100 g of it is present initially, how much remains after 18 hours?
▶️ Answer / Explanation
Step 1: \( \mathrm{t_{1/2} = 6\ h,\ t = 18\ h} \)
Step 2: Number of half-lives = \( \mathrm{t / t_{1/2} = 18 / 6 = 3} \)
Step 3: \( \mathrm{N = 100 (1/2)^3 = 100 / 8 = 12.5\ g} \)
Final Answer: 12.5 g remains after 18 hours.
Example
The activity of a radioactive isotope drops from 800 Bq to 100 Bq in 12 days. Find its half-life.
▶️ Answer / Explanation
Step 1: \( \mathrm{N_0 = 800,\ N = 100,\ t = 12\ days} \)
Step 2: Use \( \mathrm{N = N_0 (1/2)^{t/t_{1/2}}} \)
\( \mathrm{100/800 = (1/2)^{12/t_{1/2}}} \)
\( \mathrm{1/8 = (1/2)^{12/t_{1/2}}} \Rightarrow (1/2)^3 = (1/2)^{12/t_{1/2}} \)
\( \mathrm{12/t_{1/2} = 3 \Rightarrow t_{1/2} = 4\ days} \)
Final Answer: Half-life = 4 days.
Example
A sample of cobalt-60 has a half-life of 5.27 years. How long will it take for its activity to fall to 10% of its original value?
▶️ Answer / Explanation
Step 1: \( \mathrm{N/N_0 = 0.10,\ t_{1/2} = 5.27\ years} \)
Step 2: Use \( \mathrm{N = N_0 (1/2)^{t/t_{1/2}}} \)
\( \mathrm{0.10 = (1/2)^{t/5.27}} \)
Take logs: \( \mathrm{\log(0.10) = \dfrac{t}{5.27} \log(1/2)} \)
\( \mathrm{t = 5.27 \times \dfrac{\log(0.10)}{\log(0.5)} = 5.27 \times 3.32 = 17.5\ years} \)
Final Answer: It takes approximately 17.5 years for activity to fall to 10% of the original.