IB MYP 4-5 Chemistry -Hydrocarbons- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Link- Study Notes
Key Concepts
- Hydrocarbons — Alkanes, Alkenes, and Alkynes
Hydrocarbons — Alkanes, Alkenes, and Alkynes
Hydrocarbons — Alkanes, Alkenes, and Alkynes
Hydrocarbons are organic compounds composed entirely of carbon (C) and hydrogen (H) atoms. They form the basic framework for all organic compounds and can exist as gases, liquids, or solids depending on molecular size.
Classification of Hydrocarbons
Hydrocarbons are broadly divided into three main categories based on the type of bonding between carbon atoms:
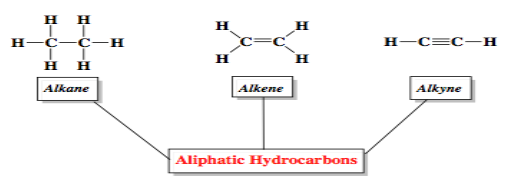
| Type | Bond Type | General Formula | Example |
|---|---|---|---|
| Alkanes | Single bonds only (C–C) | \( \mathrm{C_{n}H_{2n+2}} \) | Methane (\( \mathrm{CH_4} \)) |
| Alkenes | At least one double bond (C=C) | \( \mathrm{C_{n}H_{2n}} \) | Ethene (\( \mathrm{C_2H_4} \)) |
| Alkynes | At least one triple bond (C≡C) | \( \mathrm{C_{n}H_{2n-2}} \) | Ethyne (\( \mathrm{C_2H_2} \)) |
Alkanes (Saturated Hydrocarbons)![]()
- Contain only single covalent bonds between carbon atoms.
- Each carbon atom is fully saturated with hydrogen atoms.
- General formula: \( \mathrm{C_{n}H_{2n+2}} \).
- Examples: Methane, Ethane, Propane, Butane.
Properties:
- Nonpolar → insoluble in water.
- Low reactivity (only undergo combustion and substitution).
- Burn with a clean blue flame (complete combustion).
Combustion Reaction:
\( \mathrm{CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O + energy} \)
Substitution Reaction (with halogens):
\( \mathrm{CH_4 + Cl_2 \xrightarrow{UV} CH_3Cl + HCl} \)
Alkenes (Unsaturated Hydrocarbons)![]()
- Contain at least one double bond (C=C).
- More reactive than alkanes because of the double bond.
- General formula: \( \mathrm{C_{n}H_{2n}} \).
- Examples: Ethene, Propene, Butene.
Addition Reactions:
\( \mathrm{C_2H_4 + H_2 \xrightarrow{Ni} C_2H_6} \)
(Hydrogenation: double bond breaks, hydrogen atoms add)
Test for Unsaturation:
- Alkenes decolorize bromine water (from orange to colorless).
- Alkanes do not affect bromine water.
Combustion Reaction:
\( \mathrm{C_2H_4 + 3O_2 \rightarrow 2CO_2 + 2H_2O} \)
Alkynes (Unsaturated Hydrocarbons with Triple Bonds)
- Contain at least one triple bond (C≡C).
- General formula: \( \mathrm{C_{n}H_{2n-2}} \).
- More reactive than alkenes.
- Examples: Ethyne (acetylene), Propyne.
Combustion Reaction:
\( \mathrm{2C_2H_2 + 5O_2 \rightarrow 4CO_2 + 2H_2O} \)
Usage:
- Ethyne (\( \mathrm{C_2H_2} \)) is used in oxy-acetylene welding due to its high flame temperature.
Comparison of Alkanes, Alkenes, and Alkynes
| Property | Alkanes | Alkenes | Alkynes |
|---|---|---|---|
| Bond Type | Single (C–C) | Double (C=C) | Triple (C≡C) |
| General Formula | \( \mathrm{C_{n}H_{2n+2}} \) | \( \mathrm{C_{n}H_{2n}} \) | \( \mathrm{C_{n}H_{2n-2}} \) |
| Reactivity | Least reactive | Moderate | Most reactive |
| Bromine Test | No change | Decolorizes bromine | Decolorizes bromine |
Example
Write the molecular formula and structure of propane.
▶️ Answer / Explanation
Step 1: Propane is an alkane → general formula \( \mathrm{C_{n}H_{2n+2}} \).
Step 2: For n = 3 → \( \mathrm{C_3H_8} \).
Step 3: Structure: \( \mathrm{CH_3–CH_2–CH_3} \).
Final Answer: Molecular formula = \( \mathrm{C_3H_8} \); structure = \( \mathrm{CH_3CH_2CH_3} \).
Example
How can you distinguish between ethane and ethene using bromine water?
▶️ Answer / Explanation
Step 1: Ethane (alkane) has only single bonds and does not react with bromine water.
Step 2: Ethene (alkene) has a double bond and decolorizes bromine water by addition reaction.
Final Answer: Bromine water remains orange with ethane but becomes colorless with ethene.
Example
Calculate the volume of oxygen required for complete combustion of 1 mol of ethyne (\( \mathrm{C_2H_2} \)) and the products formed.
▶️ Answer / Explanation
Step 1: Balanced equation: \( \mathrm{2C_2H_2 + 5O_2 \rightarrow 4CO_2 + 2H_2O} \)
Step 2: 2 mol \( \mathrm{C_2H_2} \) require 5 mol \( \mathrm{O_2} \).
Step 3: 1 mol \( \mathrm{C_2H_2} \) requires \( \mathrm{2.5\ mol\ O_2} \).
Step 4: Volume of \( \mathrm{O_2} = 2.5 \times 22.4 = 56.0\ L \) (at STP).
Final Answer: 1 mol of ethyne needs 56 L of oxygen and produces \( \mathrm{2CO_2} \) and \( \mathrm{H_2O} \).
