IB MYP 4-5 Chemistry -Introduction to organic compounds- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Introduction to organic compounds- Study Notes
Key Concepts
- Introduction to organic compounds
Introduction to organic compounds
Introduction to Organic Compounds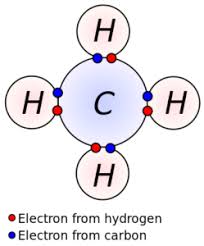
Organic compounds are chemical compounds that contain carbon atoms bonded mainly to hydrogen, oxygen, or other elements such as nitrogen, sulfur, and halogens. They form the basis of all living matter and many synthetic materials.
Origin and Meaning
- The term “organic” originally meant compounds derived from living organisms.
- Modern chemistry defines organic compounds by their carbon-based structure, not their biological source.
- Carbon’s unique ability to form four covalent bonds and long chains (catenation) makes organic chemistry incredibly diverse.
General Definition: Organic chemistry is the study of the structure, properties, composition, reactions, and synthesis of carbon-containing compounds.
Why Carbon is Special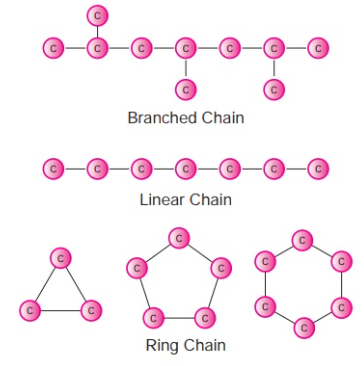
- Tetravalency: Carbon forms four covalent bonds with other atoms (e.g., H, O, N, Cl).
- Catenation: Carbon can bond to itself to form long chains, branched structures, or rings.
- Formation of multiple bonds: Carbon forms single, double, or triple bonds.
- Bond strength: Carbon–carbon and carbon–hydrogen bonds are strong and stable, allowing complex molecules to exist.
Examples:
- Methane: \( \mathrm{CH_4} \)
- Ethanol: \( \mathrm{C_2H_5OH} \)
- Glucose: \( \mathrm{C_6H_{12}O_6} \)
Classification of Organic Compounds
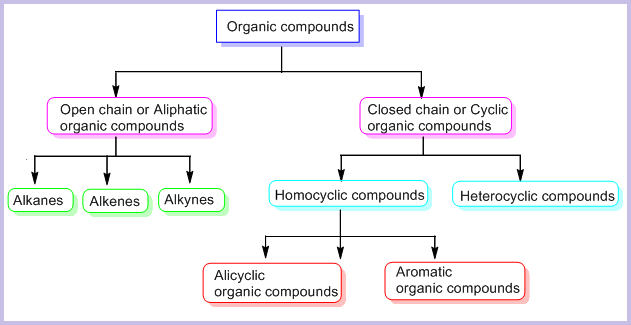
| Type | Description | Examples |
|---|---|---|
| Open-Chain (Acyclic) | Contain straight or branched carbon chains. | \( \mathrm{CH_3CH_2CH_3} \) (propane) |
| Closed-Chain (Cyclic) | Carbon atoms form rings (may be aromatic or non-aromatic). | \( \mathrm{C_6H_6} \) (benzene) |
| Aromatic | Contain benzene rings with delocalized π electrons. | Benzene, toluene |
Functional Groups
A functional group is an atom or group of atoms that gives an organic molecule its characteristic chemical properties.
| Functional Group | Formula | Example | Class |
|---|---|---|---|
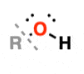
Hydroxyl | –OH | Ethanol | Alcohol |
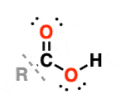
Carboxyl | –COOH | Ethanoic acid | Carboxylic acid |
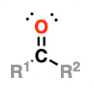
Carbonyl | C=O | Propanone | Ketone |
Importance of Organic Compounds
- In living organisms: Proteins, carbohydrates, lipids, and DNA are organic molecules essential for life.
- In medicine: Drugs, vitamins, and hormones are organic compounds.
- In daily life: Fuels (like petrol), plastics, detergents, and cosmetics are organic substances.
- In industry: Organic compounds are used to make dyes, polymers, and pesticides.
Key Characteristics of Organic Compounds
- Mostly covalent bonding
- Low melting and boiling points compared to inorganic compounds
- Insoluble in water but soluble in organic solvents
- Flammable and form complex molecular structures
Example
Identify the type of organic compound represented by \( \mathrm{CH_3CH_2OH} \) and name its functional group.
▶️ Answer / Explanation
Step 1: The compound \( \mathrm{CH_3CH_2OH} \) contains the –OH group.
Step 2: –OH is the hydroxyl group, which defines alcohols.
Final Answer: The compound is ethanol, belonging to the alcohol group.
Example
Explain why carbon can form millions of organic compounds, while silicon, which is in the same group, cannot.
▶️ Answer / Explanation
Step 1: Both carbon and silicon have four valence electrons.
Step 2: Carbon forms strong C–C and C–H bonds and can make long stable chains (catenation).
Step 3: Silicon–silicon bonds are weaker and easily broken, so silicon cannot form such diverse structures.
Final Answer: Carbon’s strong catenation and bond stability enable vast organic diversity; silicon lacks this property.
Example
Predict the type of compound formed if a hydrocarbon reacts with oxygen and state the general chemical equation.
▶️ Answer / Explanation
Step 1: Hydrocarbons are compounds of carbon and hydrogen.
Step 2: When hydrocarbons react with oxygen, they undergo combustion to form carbon dioxide and water.
Step 3: General equation: \( \mathrm{C_xH_y + O_2 \rightarrow CO_2 + H_2O + energy} \)
Final Answer: Complete combustion of hydrocarbons forms \( \mathrm{CO_2} \) and \( \mathrm{H_2O} \), releasing energy — a key reaction for fuels.