IB MYP 4-5 Chemistry -Isotopes and relative atomic mass- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Isotopes and relative atomic mass- Study Notes
Key Concepts
- Isotopes, Isobars, and Relative Atomic Mass
Isotopes, Isobars, and Relative Atomic Mass
Isotopes
Isotopes are atoms of the same element that have the same atomic number (same number of protons) but different mass numbers (different numbers of neutrons).
Each isotope of an element has identical chemical properties but slightly different physical properties because of the difference in mass.![]()
Representation
\( \mathrm{^{A}_{Z}X} \)
Where:
- \( \mathrm{X} \) = chemical symbol
- \( \mathrm{Z} \) = atomic number (same for all isotopes of an element)
- \( \mathrm{A} \) = mass number (varies between isotopes)
Examples of Isotopes
| Element | Isotopes | Protons | Neutrons | Notes & Representation |
|---|---|---|---|---|
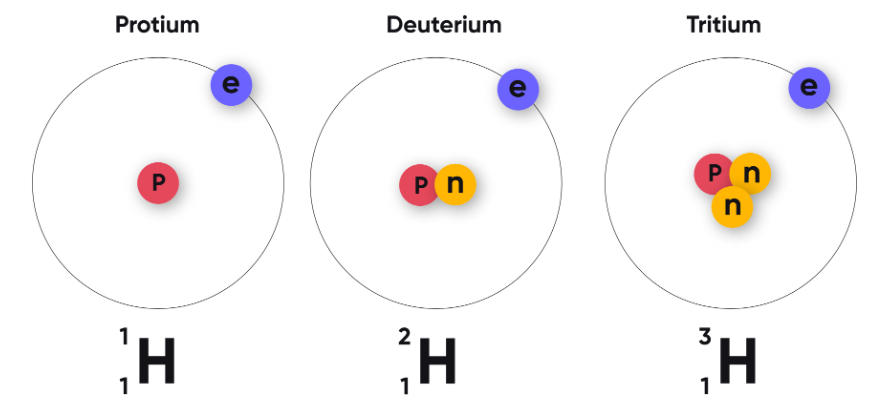
| Hydrogen | \( \mathrm{^{1}_{1}H~,~ ^{2}_{1}H~, ~^{3}_{1}H} \) | 1 | 0, 1, 2 |
Protium, Deuterium, Tritium |
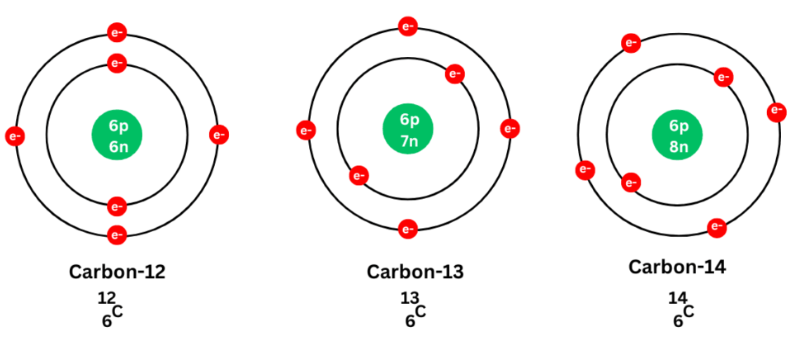
| Carbon | \( \mathrm{^{12}_{6}C~,~ ^{13}_{6}C~,~ ^{14}_{6}C} \) | 6 | 6, 7, 8 |
\( \mathrm{^{14}C} \) is radioactive (used in carbon dating) |
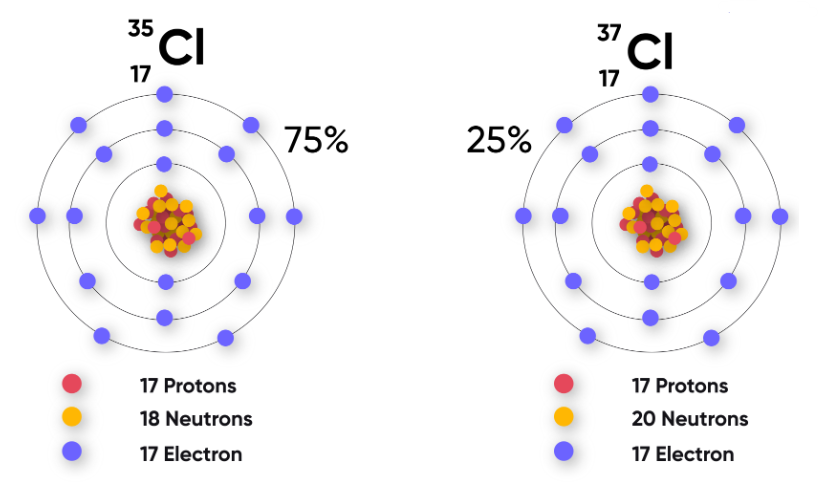
| Chlorine | \( \mathrm{^{35}_{17}Cl~,~ ^{37}_{17}Cl} \) | 17 | 18, 20 |
Exist in ratio 3:1 in nature |
Characteristics of Isotopes![]()
- Same number of protons and electrons → same chemical behavior.
- Different number of neutrons → different mass number → slightly different physical properties.
- Some isotopes are radioactive and unstable.
Examples of Use
| Isotope | Application |
|---|---|
| \( \mathrm{^{14}C} \) | Used in carbon dating (to find age of fossils) |
| \( \mathrm{^{131}I} \) | Used in medicine to diagnose and treat thyroid disorders |
| \( \mathrm{^{60}Co} \) | Used in cancer radiotherapy |
Isobars
Isobars are atoms of different elements that have the same mass number but different atomic numbers.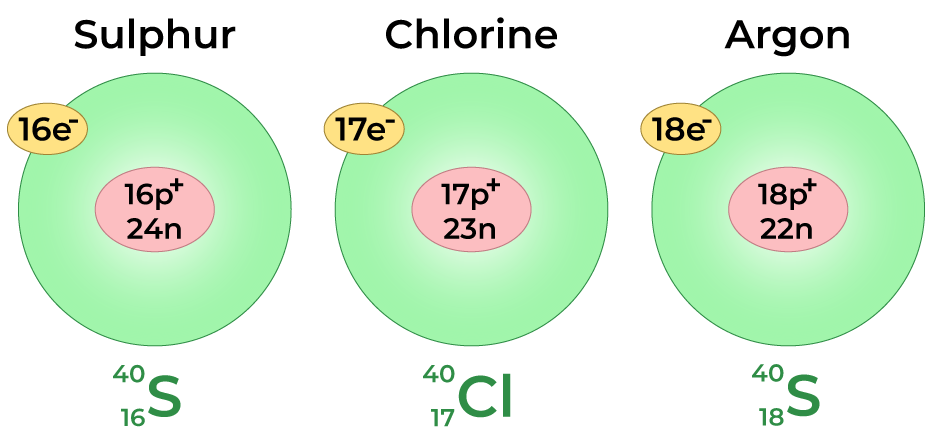
They have the same total number of nucleons (protons + neutrons) but belong to different elements.
Example: \( \mathrm{^{40}_{20}Ca} \) and \( \mathrm{^{40}_{18}Ar} \)
- Both have a mass number of 40 but different numbers of protons.
Characteristics of Isobars
- Different atomic number → different elements.
- Same mass number → total nucleons equal.
- Different chemical properties (different electrons).
Difference Between Isotopes and Isobars
| Property | Isotopes | Isobars |
|---|---|---|
| Definition | Atoms of same element with different mass numbers | Atoms of different elements with same mass number |
| Atomic Number | Same | Different |
| Mass Number | Different | Same |
| Chemical Properties | Same | Different |
| Example | \( \mathrm{^{12}_{6}C} \) and \( \mathrm{^{14}_{6}C} \) | \( \mathrm{^{40}_{20}Ca} \) and \( \mathrm{^{40}_{18}Ar} \) |
Relative Atomic Mass (\( \mathrm{A_r} \))
The relative atomic mass of an element is the average mass of its atoms compared to 1/12 the mass of a carbon-12 atom.
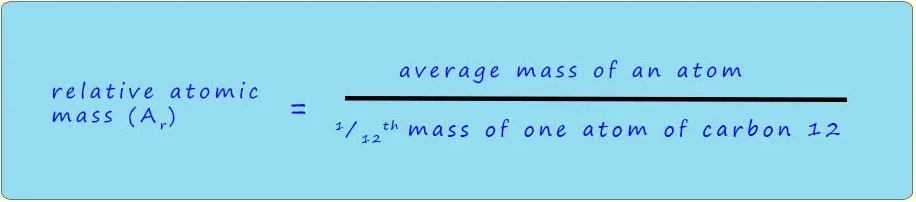
Why Average? Because most elements exist as a mixture of isotopes, the relative atomic mass is a weighted average of all isotopes based on their natural abundance.
Calculation of Relative Atomic Mass
If an element has two isotopes:
\( \mathrm{A_r = \dfrac{(mass_1 \times abundance_1) + (mass_2 \times abundance_2)}{100}} \)
Example: Chlorine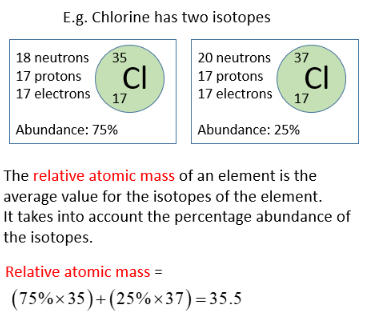
Chlorine has two isotopes:
- \( \mathrm{^{35}Cl} \) → 75% abundance
- \( \mathrm{^{37}Cl} \) → 25% abundance
Relative atomic mass:
\( \mathrm{A_r = \dfrac{(35 \times 75) + (37 \times 25)}{100} = \dfrac{2625 + 925}{100} = 35.5} \)
Answer: \( \mathrm{A_r(Cl) = 35.5} \)
Interpretation: The atomic mass of chlorine (35.5) is not a whole number because it is the weighted average of its isotopes’ masses.
Key Differences
| Term | Definition | Example |
|---|---|---|
| Isotope | Same element, different mass numbers | \( \mathrm{^{12}C, ^{14}C} \) |
| Isobar | Different elements, same mass number | \( \mathrm{^{40}Ca, ^{40}Ar} \) |
| Relative Atomic Mass | Average atomic mass of isotopes based on abundance | \( \mathrm{A_r(Cl) = 35.5} \) |
Example :
What are isotopes? Why do isotopes of an element have similar chemical properties?
▶️ Answer / Explanation
Step 1: Isotopes have the same number of protons and electrons.
Step 2: Chemical reactions depend on electron arrangement, not neutrons.
Final Answer: Isotopes of an element have identical chemical properties because their electron configurations are the same.
Example :
Calculate the relative atomic mass of copper if 69.1% is \( \mathrm{^{63}Cu} \) and 30.9% is \( \mathrm{^{65}Cu} \).
▶️ Answer / Explanation
Step 1: \( \mathrm{A_r = \dfrac{(63 \times 69.1) + (65 \times 30.9)}{100}} \)
Step 2: \( \mathrm{A_r = \dfrac{4353.3 + 2008.5}{100} = 63.6} \)
Final Answer: \( \mathrm{A_r(Cu) = 63.6} \)
Example:
Explain why the relative atomic mass of chlorine is not a whole number even though individual isotopes have whole-number masses.
▶️ Answer / Explanation
Step 1: Chlorine exists as two isotopes: \( \mathrm{^{35}Cl} \) and \( \mathrm{^{37}Cl} \).
Step 2: Their natural abundances (75% and 25%) cause an average atomic mass of 35.5.
Step 3: Weighted averages rarely result in whole numbers.
Final Answer: The atomic mass of chlorine (35.5) is an average of its isotopes’ masses, not the mass of one atom.