IB MYP 4-5 Chemistry -IUPAC Naming of Organic Compounds- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -IUPAC Naming of Organic Compounds- Study Notes
Key Concepts
- IUPAC Naming of Organic Compounds
IUPAC Naming of Organic Compounds
IUPAC Naming of Organic Compounds
The International Union of Pure and Applied Chemistry (IUPAC) system provides a standardized method for naming organic compounds. This ensures that every chemical name corresponds to one specific structure and avoids confusion.
Every organic compound name consists of three parts — the prefix, root, and suffix.
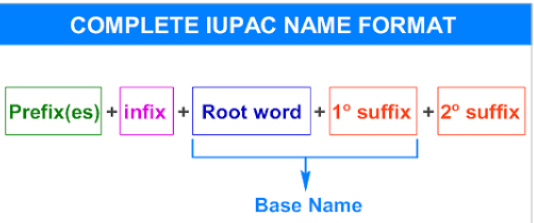
Parts of an IUPAC Name
| Part | Description | Example |
|---|---|---|
| Prefix | Indicates substituents or side chains (e.g., methyl, chloro) | 2-methyl, 3-chloro |
| Root | Indicates the number of carbon atoms in the longest continuous chain | meth-, eth-, prop-, but-, pent-, hex-, etc. |
| Suffix | Indicates the main functional group | -ane (alkane), -ene (alkene), -ol (alcohol), -oic acid (acid) |
Steps in IUPAC Naming 
- Identify the longest continuous carbon chain — this gives the root name (meth-, eth-, prop-, etc.).
- Number the carbon atoms so that the main functional group or double bond gets the lowest possible number.
- Identify and name substituents (branches) such as alkyl groups (–CH₃, –C₂H₅) or halogens (–Cl, –Br).
- Assign numbers to substituents according to their position on the chain.
- Combine all parts in the order: Prefix (branches/substituents) → Root (main chain) → Suffix (functional group).
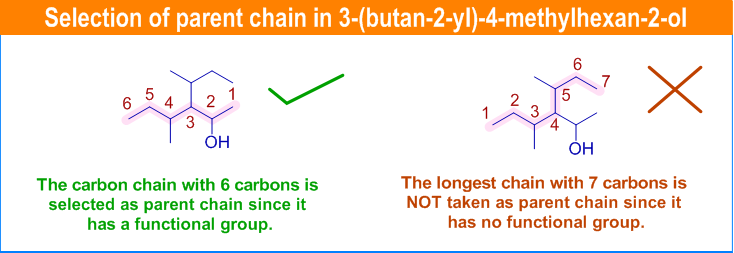
Root Names Based on Carbon Chain Length
| Number of Carbons | Root Name | Example Compound |
|---|---|---|
| 1 | meth- | Methane (\( \mathrm{CH_4} \)) |
| 2 | eth- | Ethane (\( \mathrm{C_2H_6} \)) |
| 3 | prop- | Propane (\( \mathrm{C_3H_8} \)) |
| 4 | but- | Butane (\( \mathrm{C_4H_{10}} \)) |
| 5 | pent- | Pentane (\( \mathrm{C_5H_{12}} \)) |
| 6 | hex- | Hexane (\( \mathrm{C_6H_{14}} \)) |
Primary Suffix — Type of Carbon Bond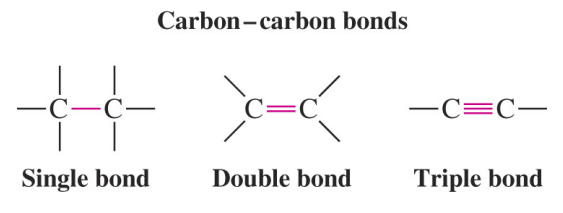
- -ane: All single bonds (alkanes)
- -ene: One or more double bonds (alkenes)
- -yne: One or more triple bonds (alkynes)
Example:
\( \mathrm{CH_3-CH_2-CH_3} \) → Propane (all single bonds)
\( \mathrm{CH_2=CH-CH_3} \) → Propene (one double bond)
\( \mathrm{CH \equiv C-CH_3} \) → Propyne (one triple bond)
Functional Groups and Their Suffixes
| Functional Group | General Formula | Suffix | Example |
|---|---|---|---|
| Alcohol | –OH | -ol | Ethanol (\( \mathrm{CH_3CH_2OH} \)) |
| Aldehyde | –CHO | -al | Ethanal (\( \mathrm{CH_3CHO} \)) |
| Ketone | >C=O | -one | Propanone (\( \mathrm{CH_3COCH_3} \)) |
| Carboxylic Acid | –COOH | -oic acid | Ethanoic acid (\( \mathrm{CH_3COOH} \)) |
| Ester | –COOR | -oate | Methyl ethanoate |
Prefixes for Substituents (Side Chains)
- Methyl (–CH₃)
- Ethyl (–C₂H₅)
- Propyl (–C₃H₇)
- Chloro (–Cl), Bromo (–Br), Nitro (–NO₂)
If there are multiple identical substituents, use numerical prefixes:
- 2 → di-
- 3 → tri-
- 4 → tetra-
Example: \( \mathrm{CH_3-CH(CH_3)-CH_3} \) → 2-methylpropane
Rules for Numbering
- Number the chain from the end nearest the functional group or substituent.
- If two groups are at equal distances, give priority to the one with alphabetical order (e.g., bromo before methyl).
- Indicate position numbers before each substituent (e.g., 2-butanol, 3-methylpentane).
Example
Write the IUPAC name of \( \mathrm{CH_3CH_2CH_3} \).
▶️ Answer / Explanation
Step 1: Three carbons → root = prop-.
Step 2: All single bonds → suffix = -ane.
Final Answer: Propane.
Example
Write the IUPAC name of \( \mathrm{CH_3CH(CH_3)CH_2CH_3} \).
▶️ Answer / Explanation
Step 1: Longest chain → 4 carbons → butane.
Step 2: One –CH₃ branch on the 2nd carbon → 2-methyl.
Step 3: Combine → 2-methylbutane.
Final Answer: 2-methylbutane.
Example
Write the IUPAC name of \( \mathrm{CH_3CH_2CH(OH)CH_3} \).
▶️ Answer / Explanation
Step 1: Longest chain → 4 carbons → but-.
Step 2: Functional group –OH on carbon 2 → -2-ol.
Step 3: Combine → butan-2-ol.
Final Answer: Butan-2-ol.
