IB MYP 4-5 Chemistry -Metallic bonding and alloys- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Metallic bonding and alloys- Study Notes
Key Concepts
- Metallic Bonding and Alloys
Metallic Bonding and Alloys
Metallic Bonding and Alloys
Metallic bonding is the type of chemical bonding that occurs between metal atoms. It involves a lattice of positive metal ions surrounded by a ‘sea’ of delocalized electrons that move freely throughout the structure.
This bond explains the unique physical properties of metals such as electrical conductivity, malleability, ductility, and metallic lustre.
Metallic Bonding![]()
Structure:
- Metal atoms lose their outer electrons → form positive ions.
- These electrons become delocalized (not attached to any one atom).
- The electrostatic attraction between the metal cations and the delocalized electrons holds the structure together.
Structure summary: \( \mathrm{Metal\ lattice = Positive\ ions + Delocalized\ electrons} \)
Example: In sodium metal (\( \mathrm{Na} \)), each atom loses one electron to form \( \mathrm{Na^+} \). The released electrons move freely throughout the lattice, forming metallic bonds.
Diagram description (MYP-level): Visualize rows of positive metal ions (\( \mathrm{Na^+} \)) arranged closely together, surrounded by a ‘cloud’ of mobile electrons. The electrons can move freely between ions — creating a strong yet flexible structure.
![]()
Properties of Metallic Substances (and Their Reasons):
| Property | Explanation |
|---|---|
| High Electrical Conductivity | Delocalized electrons move freely and carry electric current. |
| Thermal Conductivity | Free electrons transfer heat energy efficiently through collisions. |
| Malleability & Ductility | Metal ions can slide past each other without breaking bonds because electrons hold them together in all directions. |
| High Melting and Boiling Points | Strong attraction between ions and electrons requires large energy to break. |
| Metallic Lustre (Shine) | Free electrons reflect light from the surface, giving metals their shiny appearance. |
Alloys
An alloy is a mixture of two or more elements, at least one of which is a metal, made to improve strength, hardness, or resistance to corrosion.
Formation of Alloys:
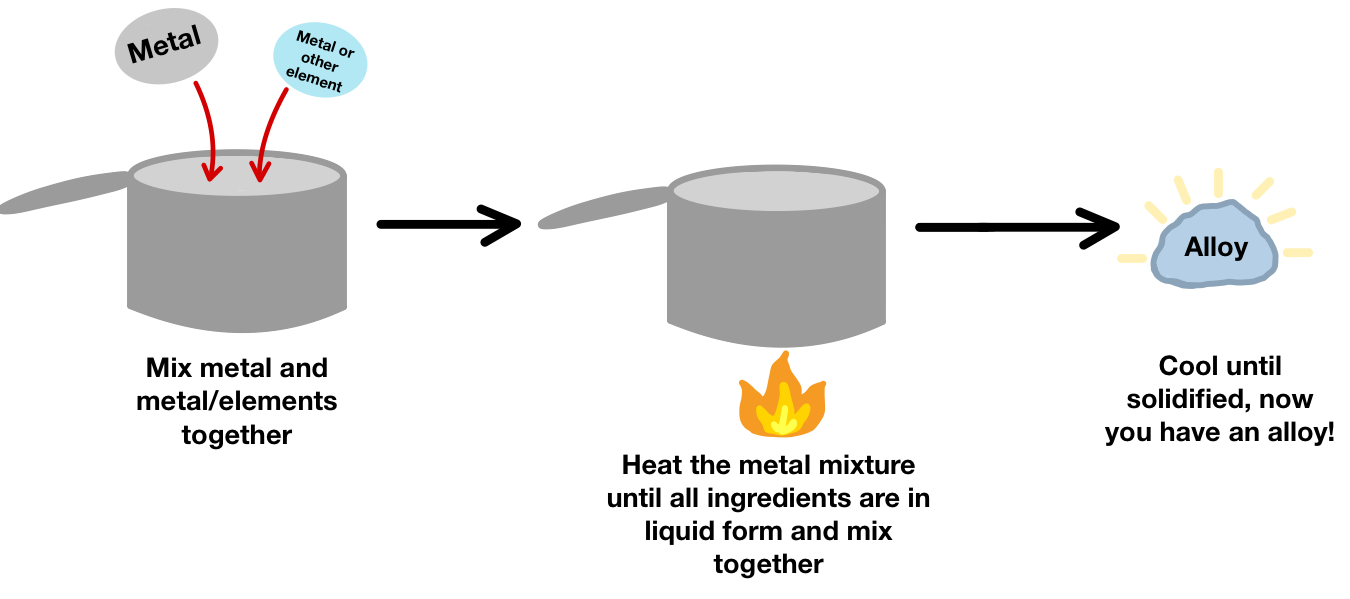
- Atoms of the added element(s) are different in size from the main metal’s atoms.
- This distorts the regular metal lattice, making it more difficult for layers of atoms to slide over each other.
- As a result, alloys are usually stronger and harder than pure metals.
Common Examples of Alloys:
| Alloy | Main Elements | Properties / Uses |
|---|---|---|
| Brass | Copper + Zinc | Strong, corrosion-resistant; used in musical instruments and fittings. |
| Bronze | Copper + Tin | Harder than copper; used in statues and medals. |
| Steel | Iron + Carbon | Hard, strong; used in buildings, vehicles, and machinery. |
| Stainless Steel | Iron + Chromium + Nickel | Resists corrosion; used in kitchenware and medical instruments. |
Effect of Alloying on Structure:
- Pure metals have regular, identical layers of atoms that slide easily → soft and malleable.
- In alloys, atoms of different sizes distort the lattice → layers cannot slide easily → harder and stronger.
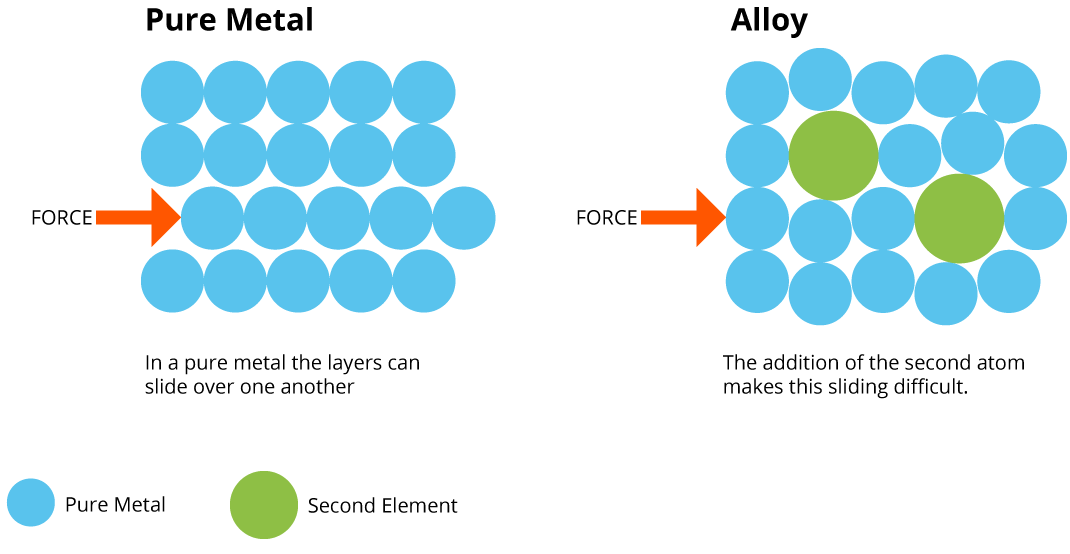
Visual Concept (MYP-level): Pure metal = neat, uniform rows of atoms. Alloy = irregular atom sizes break up smooth layers, strengthening the structure.
Relationship Between Bonding, Structure, and Properties
| Aspect | Pure Metals | Alloys |
|---|---|---|
| Structure | Regular layers of atoms | Irregular, distorted layers (due to different atom sizes) |
| Bonding Type | Metallic bonding (delocalized electrons) | Same, but lattice distortion strengthens bonds |
| Malleability | High (layers slide easily) | Reduced (layers cannot slide easily) |
| Hardness | Soft | Hard and strong |
Example :
Explain why metals conduct electricity.
▶️ Answer / Explanation
Step 1: Metallic bonding produces delocalized electrons.
Step 2: These electrons move freely through the metal lattice.
Final Answer: Metals conduct electricity because delocalized electrons can carry charge throughout the structure.
Example:
Why is an alloy such as steel harder than pure iron?
▶️ Answer / Explanation
Step 1: Pure iron has atoms of the same size that form smooth layers → easy to slide.
Step 2: In steel, carbon atoms are smaller and fit between iron atoms.
Step 3: This distorts the lattice → layers cannot slide easily.
Final Answer: Steel is harder than iron because carbon atoms strengthen and distort the metal lattice, preventing slippage.
Example :
Explain, in terms of metallic bonding, why metals have both high melting points and are malleable.
▶️ Answer / Explanation
Step 1: Strong electrostatic forces exist between metal ions and delocalized electrons → require high energy to break → high melting point.
Step 2: When a metal is hammered, layers of ions can slide over each other.
Step 3: The delocalized electrons adjust and maintain attraction → structure does not break.
Final Answer: Metals are both strong and malleable because metallic bonds are strong yet non-directional — allowing ions to move without breaking the bond.
