IB MYP 4-5 Chemistry -Metals, non-metals, noble gases, and transition metals- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Metals, non-metals, noble gases, and transition metals- Study Notes
Key Concepts
- Metals, Non-metals, Noble Gases, and Transition Metals
Metals, Non-metals, Noble Gases, and Transition Metals
Metals, Non-metals, Noble Gases, and Transition Metals
All elements in the periodic table can be broadly classified into metals, non-metals, noble gases, and transition metals based on their physical and chemical properties.
These categories explain how elements conduct electricity, form compounds, and react with other substances.
Metals
Metals are elements that easily lose electrons to form positive ions (\( \mathrm{cations} \)). They are usually found on the left-hand side and in the center of the periodic table.
Physical Properties: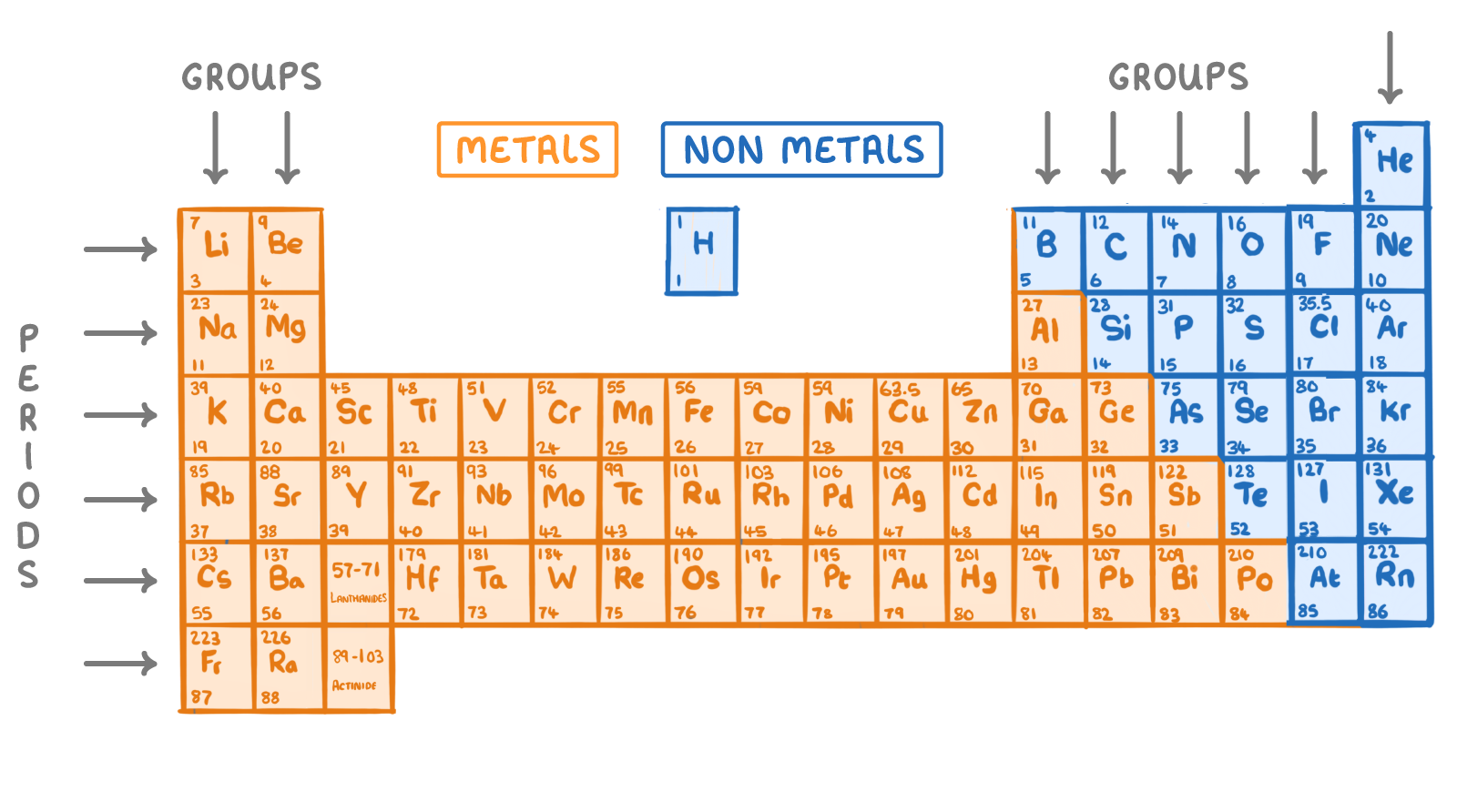
- Good conductors of heat and electricity
- High melting and boiling points
- Malleable (can be hammered into sheets)
- Ductile (can be drawn into wires)
- Have metallic lustre (shiny appearance)
- Solid at room temperature (except mercury)
- High density and strength
Chemical Properties:
- Form positive ions by losing electrons: \( \mathrm{Na \rightarrow Na^+ + e^-} \)
- React with oxygen to form basic oxides: \( \mathrm{2Mg + O_2 \rightarrow 2MgO} \)
- React with acids to release hydrogen gas: \( \mathrm{Zn + 2HCl \rightarrow ZnCl_2 + H_2} \)
Examples: Sodium (Na), Calcium (Ca), Iron (Fe), Copper (Cu), Zinc (Zn), Aluminum (Al).
Non-metals
Non-metals are elements that gain or share electrons to form negative ions (\( \mathrm{anions} \)) or covalent bonds. They are found on the right-hand side of the periodic table.
Physical Properties: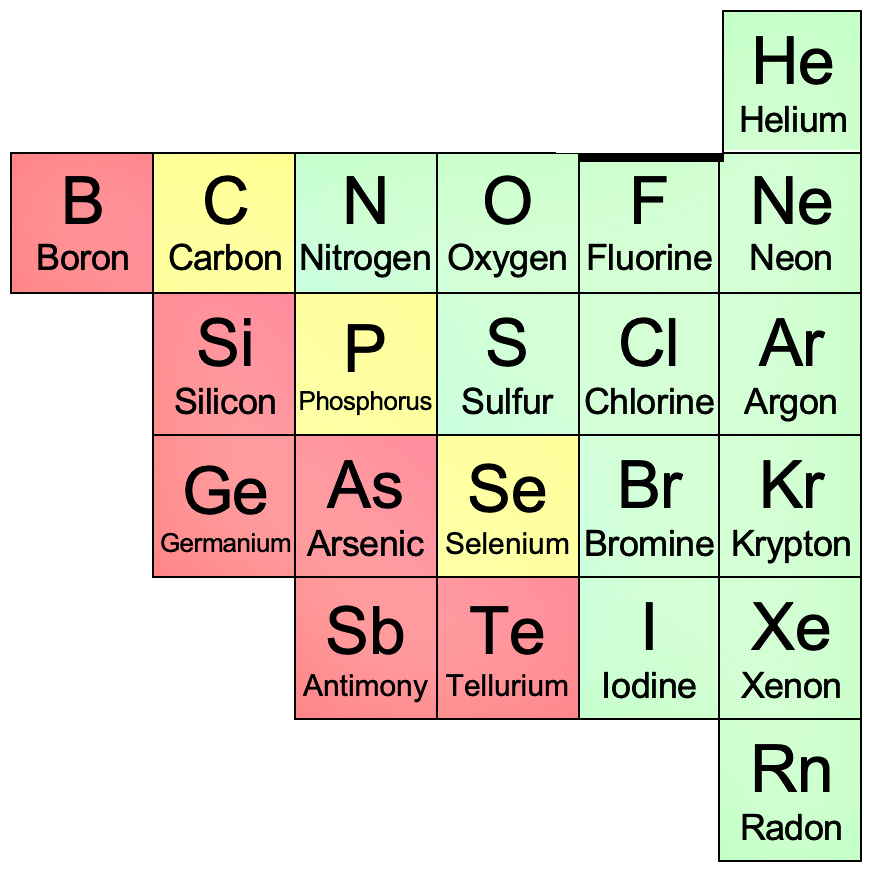
- Poor conductors of heat and electricity (insulators)
- Low melting and boiling points
- Brittle (when solid)
- No metallic lustre (dull appearance)
- Can be solid, liquid, or gas at room temperature
Chemical Properties:
- Form negative ions by gaining electrons: \( \mathrm{Cl + e^- \rightarrow Cl^-} \)
- React with oxygen to form acidic oxides: \( \mathrm{C + O_2 \rightarrow CO_2} \)
- Combine with metals to form ionic compounds: \( \mathrm{2Na + Cl_2 \rightarrow 2NaCl} \)
Examples: Oxygen (O), Nitrogen (N), Sulfur (S), Chlorine (Cl), Carbon (C).
Noble Gases
Noble gases are elements in Group 18 that have completely filled electron shells. They are chemically inert and do not readily react with other elements.
Properties: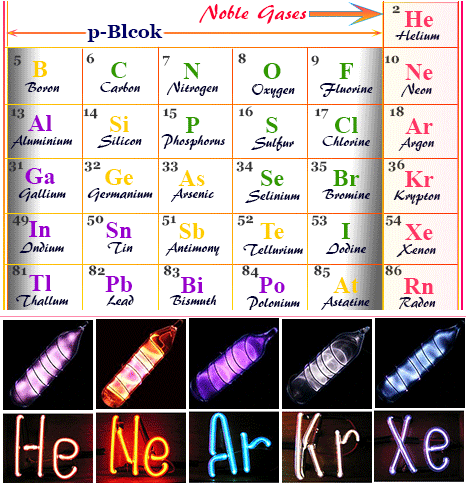
- Exist as monoatomic gases (single atoms)
- Colorless, odorless, tasteless
- Low density and low boiling points
- Very stable electron configuration (octet/duplet)
- Do not form compounds easily (inert gases)
Electron Configuration:
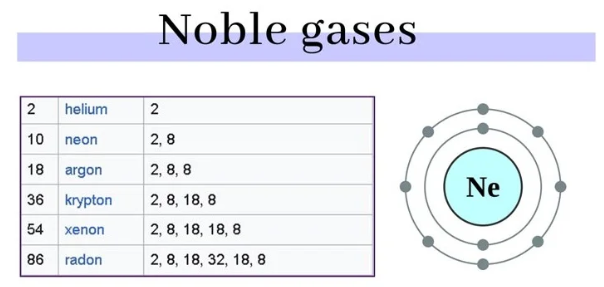
Uses:
- Helium → balloons and airships
- Neon → advertising signs
- Argon → welding and light bulbs
Transition Metals
Transition metals are elements found in the middle block of the periodic table (Groups 3–12). They are metals with variable oxidation states and form colored compounds.
Physical Properties:
- Good conductors of heat and electricity
- High melting and boiling points
- Hard and dense metals
- Form alloys (mixtures of metals)
- Show metallic lustre
Chemical Properties:
- Form colored ions and compounds (e.g., \( \mathrm{Cu^{2+}} \) → blue, \( \mathrm{Fe^{3+}} \) → yellow/brown)
- Exhibit variable valency (e.g., \( \mathrm{Fe^{2+}} \) and \( \mathrm{Fe^{3+}} \))
- Act as catalysts (e.g., \( \mathrm{Fe} \) in ammonia production, \( \mathrm{Ni} \) in hydrogenation)
Examples: Iron (Fe), Copper (Cu), Zinc (Zn), Nickel (Ni), Chromium (Cr), Silver (Ag).
Comparison Table: Metals vs Non-metals vs Noble Gases vs Transition Metals
| Property | Metals | Non-metals | Noble Gases | Transition Metals |
|---|---|---|---|---|
| State at Room Temp. | Mostly solid (except Hg) | Solid, liquid, or gas | Gas | Solid (except Hg) |
| Electrical Conductivity | Good | Poor | Poor | Excellent |
| Lustre | Shiny | Dull | Colorless gas | Highly lustrous |
| Type of Oxide | Basic | Acidic | None | Mostly basic or amphoteric |
| Ion Formation | Positive ions (cations) | Negative ions (anions) | None (inert) | Variable oxidation states |
| Reactivity | Moderate to high | Varies (often high) | Very low | Moderate |
Example:
Why are metals good conductors of electricity while non-metals are not?
▶️ Answer / Explanation
Step 1: Metals have free-moving electrons in their structure.
Step 2: These delocalized electrons carry electric current easily.
Step 3: Non-metals have tightly bound electrons that cannot move freely.
Final Answer: Metals conduct electricity because of free electrons; non-metals do not because their electrons are localized.
Example :
Explain why noble gases do not readily form compounds.
▶️ Answer / Explanation
Step 1: Noble gases have completely filled electron shells (stable configuration).
Step 2: They neither gain nor lose electrons easily.
Final Answer: Noble gases are chemically inert because they already have a stable electron configuration (octet or duplet).
Example :
Transition metals often form colored compounds, unlike most main-group metals. Explain why.
▶️ Answer / Explanation
Step 1: Transition metals have partially filled d-orbitals.
Step 2: When light passes through, electrons in the d-orbitals absorb specific wavelengths to move to higher energy levels.
Step 3: The remaining light reflected gives the compound its color.
Final Answer: Colored compounds of transition metals arise due to electronic transitions within d-orbitals when they absorb visible light energy.
