IB MYP 4-5 Chemistry -Moles, mass, and molar relationships- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Moles, mass, and molar relationships- Study Notes
Key Concepts
- Moles, Mass, and Molar Relationships
Moles, Mass, and Molar Relationships
Moles, Mass, and Molar Relationships
The mole is a unit used to measure the amount of a substance. It links the number of microscopic particles (atoms, molecules, or ions) to the measurable macroscopic quantities like mass and volume.
Key Idea: The mole connects the mass of a substance with the number of particles it contains using the molar mass (mass of one mole of a substance).
The Relationship Between Moles and Mass
Formula:
\( \mathrm{n = \dfrac{m}{M}} \)
- \( \mathrm{n} \) = number of moles (mol)
- \( \mathrm{m} \) = mass of the substance (g)
- \( \mathrm{M} \) = molar mass (g/mol)
Rearranged Forms:
- \( \mathrm{m = nM} \)
- \( \mathrm{M = \dfrac{m}{n}} \)
Meaning: This formula shows that the number of moles depends directly on the mass of the substance and inversely on its molar mass.
The Relationship Between Moles and Number of Particles
Formula:
\( \mathrm{n = \dfrac{N}{N_A}} \)
- \( \mathrm{N} \) = number of particles (atoms, molecules, or ions)
- \( \mathrm{N_A} \) = Avogadro’s constant = \( \mathrm{6.022 \times 10^{23}\ particles\ mol^{-1}} \)
Rearranged Forms:
- \( \mathrm{N = n \times N_A} \)
- \( \mathrm{n = \dfrac{N}{N_A}} \)
Meaning: One mole of any substance contains \( \mathrm{6.022 \times 10^{23}} \) particles. This constant allows us to convert between microscopic particle counts and macroscopic moles.
The Relationship Between Moles and Gas Volume (for Gases at STP)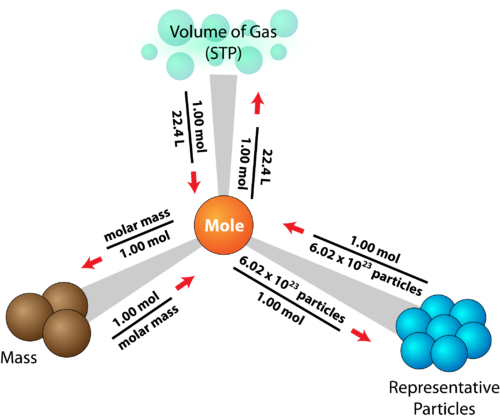
At standard temperature and pressure (STP) — 0°C and 1 atm 1 mole of any gas occupies:
\( \mathrm{22.4\ dm^3 = 22,400\ cm^3} \)
Formula:
\( \mathrm{n = \dfrac{V}{V_m}} \)
- \( \mathrm{V} \) = volume of gas (dm³)
- \( \mathrm{V_m} \) = molar volume of gas (22.4 dm³/mol at STP)
Meaning: Equal volumes of all gases (at the same temperature and pressure) contain equal numbers of particles — an application of Avogadro’s Law.
Molar Relationships in Chemical Reactions
A balanced chemical equation shows the mole ratio of reactants and products — the proportional relationship between substances in a reaction.
Example: \( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \)
- 2 moles of hydrogen react with 1 mole of oxygen → 2 moles of water.
- Mole ratio = \( \mathrm{2:1:2} \)

Steps to Use Molar Relationships:
- Write and balance the chemical equation.
- Identify the known and unknown quantities.
- Convert mass (if given) to moles using \( \mathrm{n = \dfrac{m}{M}} \).
- Use the mole ratio from the equation to find moles of the unknown.
- Convert back to mass, volume, or particles as needed.
Summary Table: Moles, Mass, Volume, and Particles Relationships
| Quantity | Symbol | Formula | Units |
|---|---|---|---|
| Mass | \( \mathrm{m} \) | \( \mathrm{n = \dfrac{m}{M}} \) | g |
| Number of particles | \( \mathrm{N} \) | \( \mathrm{n = \dfrac{N}{N_A}} \) | — |
| Gas volume (at STP) | \( \mathrm{V} \) | \( \mathrm{n = \dfrac{V}{22.4}} \) | dm³ |
Example
Calculate the number of moles in 12 g of carbon.
▶️ Answer / Explanation
Step 1: Molar mass of carbon = 12 g/mol
Step 2: \( \mathrm{n = \dfrac{m}{M} = \dfrac{12}{12} = 1\ mol} \)
Final Answer: 12 g of carbon = 1 mole.
Example
How many molecules are present in 18 g of water (\( \mathrm{H_2O} \))?
▶️ Answer / Explanation
Step 1: Molar mass of \( \mathrm{H_2O = 18\ g/mol} \)
Step 2: \( \mathrm{n = \dfrac{18}{18} = 1\ mol} \)
Step 3: Number of molecules = \( \mathrm{n \times N_A = 1 \times 6.022 \times 10^{23}} \)
Final Answer: \( \mathrm{6.022 \times 10^{23}} \) molecules of water.
Example
How many liters of carbon dioxide at STP are produced when 4 g of methane (\( \mathrm{CH_4} \)) burns completely in oxygen?
Equation: \( \mathrm{CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O} \)
▶️ Answer / Explanation
Step 1: Molar mass of \( \mathrm{CH_4 = 16\ g/mol} \)
Step 2: \( \mathrm{n(CH_4) = \dfrac{4}{16} = 0.25\ mol} \)
Step 3: From the balanced equation, 1 mol \( \mathrm{CH_4} \) → 1 mol \( \mathrm{CO_2} \)
So, \( \mathrm{n(CO_2) = 0.25\ mol} \)
Step 4: \( \mathrm{V = n \times 22.4 = 0.25 \times 22.4 = 5.6\ dm^3} \)
Final Answer: \( \mathrm{5.6\ dm^3} \) of \( \mathrm{CO_2} \) gas is produced at STP.

