IB MYP 4-5 Chemistry -Ozone layer and CFCs- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Ozone layer and CFCs- Study Notes
Key Concepts
- Ozone Layer Depletion and CFCs (Chlorofluorocarbons)
Ozone Layer Depletion and CFCs (Chlorofluorocarbons)
Ozone Layer Depletion and CFCs (Chlorofluorocarbons)
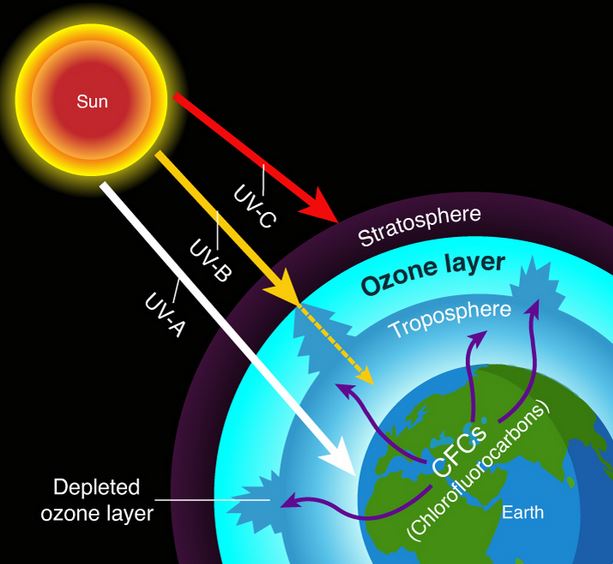
The ozone layer is a region in the stratosphere (about 15–35 km above Earth) that contains a high concentration of ozone (\( \mathrm{O_3} \)). It protects living organisms by absorbing most of the Sun’s harmful ultraviolet (UV) radiation. Depletion of this layer means thinning or reduction of ozone concentration, mainly due to man-made chemicals like chlorofluorocarbons (CFCs).
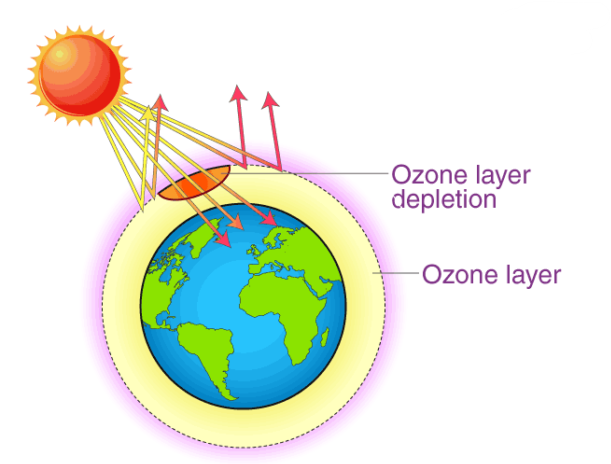
The Ozone Layer — Formation and Importance
Formation of Ozone:
Ozone is formed naturally in the stratosphere when ultraviolet (UV) radiation splits oxygen molecules:
\( \mathrm{O_2 \xrightarrow{UV} 2O} \)
\( \mathrm{O + O_2 \rightarrow O_3} \)
Natural Ozone–Oxygen Cycle:
Ozone absorbs harmful UV radiation and breaks down back into oxygen molecules and atoms:
\( \mathrm{O_3 \xrightarrow{UV} O_2 + O} \)
This continuous formation and breakdown maintain the ozone balance in the atmosphere.
Importance:
- Absorbs 97–99% of harmful UV-B and UV-C rays.
- Prevents skin cancer, cataracts, and DNA damage in organisms.
- Protects crops and aquatic ecosystems from UV harm.
What Causes Ozone Layer Depletion?
Human-made gases such as CFCs (chlorofluorocarbons), halons, and nitrous oxide (N₂O) break down ozone molecules in the stratosphere.
- CFCs: Contain chlorine (Cl), fluorine (F), and carbon (C).
- Used in refrigerators, air conditioners, aerosol sprays, and foam manufacturing.
Why CFCs are harmful:
- They are stable and non-reactive in the lower atmosphere.
- They rise into the stratosphere, where UV radiation breaks them down, releasing chlorine atoms that destroy ozone.
Chemical Reactions of Ozone Depletion by CFCs
Step 1: UV radiation breaks CFC molecules to release chlorine atoms:
\( \mathrm{CFCl_3 \xrightarrow{UV} CFCl_2 + Cl^\bullet} \)
Step 2: The chlorine atom reacts with ozone:
\( \mathrm{Cl^\bullet + O_3 \rightarrow ClO^\bullet + O_2} \)
Step 3: The chlorine monoxide reacts with another oxygen atom:
\( \mathrm{ClO^\bullet + O \rightarrow Cl^\bullet + O_2} \)
Result: One chlorine atom can destroy thousands of ozone molecules before being neutralized.
Overall Reaction:
\( \mathrm{O_3 + O \rightarrow 2O_2} \)
Other Ozone-Depleting Substances (ODS)
| Substance | Chemical Type | Source / Use | Effect on Ozone |
|---|---|---|---|
| CFCs | Chlorofluorocarbons | Aerosol sprays, refrigeration, air conditioning | Release Cl atoms that destroy ozone |
| Halons | Bromine compounds | Fire extinguishers | Bromine is even more destructive than chlorine |
| N₂O | Nitrous oxide | Fertilizers, biomass burning | Breaks down to release reactive nitrogen species |
Effects of Ozone Layer Depletion
- Increased UV radiation reaching Earth’s surface.
- Higher rates of skin cancer and cataracts in humans.
- Reduced crop yields and forest productivity.
- Damage to phytoplankton — disrupts marine food chains.
- Degradation of materials such as plastics and paints.
Ozone Hole
The term “ozone hole” refers to the seasonal thinning of the ozone layer, mainly over Antarctica during spring (September–November). Low temperatures and polar stratospheric clouds accelerate reactions that destroy ozone.
Control and Prevention Measures
- International Action: The Montreal Protocol (1987) — a global treaty to phase out ozone-depleting substances (CFCs, halons, etc.).
- Use Alternatives: Replace CFCs with hydrofluorocarbons (HFCs) or other eco-friendly refrigerants.
- Reduce Aerosol Use: Use mechanical pumps instead of CFC-propelled sprays.
- Monitor Atmospheric Ozone: Satellites track ozone levels to assess recovery.
Ozone Layer and CFCs
| Aspect | Details |
|---|---|
| Location | Stratosphere (15–35 km above Earth) |
| Function | Absorbs harmful UV radiation |
| Main Pollutants | CFCs, halons, N₂O |
| Effect | Thinning of ozone layer → more UV rays reach Earth |
| International Treaty | Montreal Protocol (1987) |
Example
What is the role of the ozone layer in the atmosphere?
▶️ Answer / Explanation
Step 1: The ozone layer absorbs harmful ultraviolet (UV) radiation from the Sun.
Step 2: This prevents damage to living cells, reducing skin cancer and eye diseases.
Final Answer: The ozone layer protects life on Earth by blocking most UV radiation.
Example
Explain how CFCs cause ozone depletion in the stratosphere using chemical equations.
▶️ Answer / Explanation
Step 1: UV light breaks CFC molecules to release chlorine atoms.
\( \mathrm{CFCl_3 \xrightarrow{UV} CFCl_2 + Cl^\bullet} \)
Step 2: Chlorine reacts with ozone.
\( \mathrm{Cl^\bullet + O_3 \rightarrow ClO^\bullet + O_2} \)
Step 3: Chlorine is regenerated and continues the cycle.
\( \mathrm{ClO^\bullet + O \rightarrow Cl^\bullet + O_2} \)
Final Answer: Chlorine atoms act as catalysts, continuously destroying ozone molecules.
Example
Discuss how international cooperation has helped in reducing ozone depletion and protecting the environment.
▶️ Answer / Explanation
Step 1: The Montreal Protocol (1987) brought countries together to phase out CFCs and other ODSs.
Step 2: Alternatives like HFCs and HCFCs were developed for refrigeration and aerosol use.
Step 3: Satellite data show gradual recovery of ozone concentration since 2000.
Final Answer: International agreements, scientific awareness, and eco-friendly alternatives have significantly reduced ozone depletion worldwide.
