IB MYP 4-5 Chemistry -Particle model and kinetic theory- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Particle Model and Kinetic Theory- Study Notes
Key Concepts
- Particle Model and Kinetic Theory
Particle Model and Kinetic Theory
Particle Model and Kinetic Theory
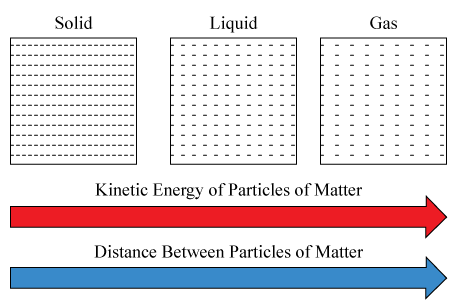
The Particle Model of Matter explains that all substances are made of tiny, discrete particles (atoms, ions, or molecules) which are in constant motion. The arrangement, movement, and energy of these particles determine the physical state (solid, liquid, or gas) and the properties of the substance.
The Kinetic Theory of Matter builds upon this idea — it states that the temperature of a substance is a measure of the average kinetic energy of its particles. As energy increases, particle motion becomes faster, leading to changes in state and physical properties.
Main Assumptions of the Particle Model
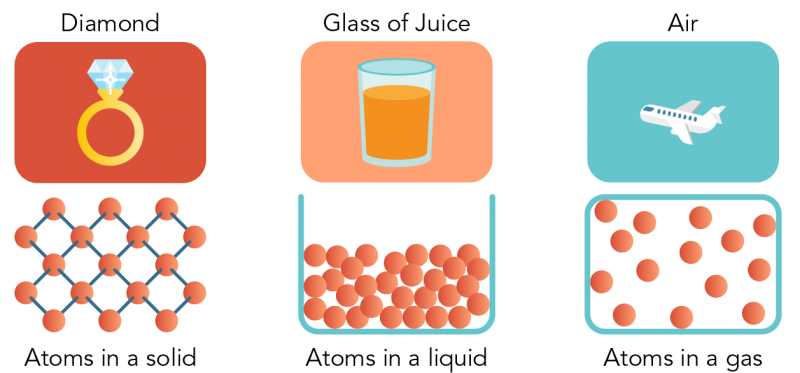
- All matter is made of extremely small particles (atoms, ions, or molecules).
- There are empty spaces between these particles.
- Particles are in constant motion — vibrating, sliding, or moving freely depending on the state.
- There are forces of attraction between particles that vary in strength depending on the substance and state.
- As temperature increases, particles move faster (gain kinetic energy).
Kinetic Theory of Matter
The Kinetic Theory explains the behavior of matter by relating the motion and energy of particles to observable properties such as temperature, pressure, and volume.
Formula for average kinetic energy per mole of gas particles:
\( \mathrm{KE_{avg} = \dfrac{3}{2}RT} \)
- \( \mathrm{KE_{avg}} \): Average kinetic energy per mole of particles
- \( \mathrm{R} \): Universal gas constant (\( \mathrm{8.314\ J\ mol^{-1}\ K^{-1}} \))
- \( \mathrm{T} \): Absolute temperature (in kelvin)
Key Relationship: \( \mathrm{KE_{avg} \propto T} \) → As temperature increases, particle kinetic energy increases linearly.
Connecting Particle Model and Kinetic Theory
| Concept | Particle Model Description | Kinetic Theory Explanation |
|---|---|---|
| Temperature | Determines how fast particles move | Proportional to the average kinetic energy of particles |
| Pressure (in gases) | Caused by particles colliding with container walls | Greater particle speed → more frequent and forceful collisions → higher pressure |
| Phase Changes | Changes occur when particle energy increases or decreases | Energy added increases kinetic motion and overcomes intermolecular forces |
Key Ideas
- The higher the temperature, the greater the particle motion and the higher the kinetic energy.
- Gases have much more particle motion than solids or liquids because of their higher energy and weaker forces.
- Pressure and volume changes in gases can be explained entirely by particle motion and collision frequency.
- At absolute zero (0 K), all molecular motion theoretically stops — the lowest possible energy state.
Example :
When the temperature of a gas increases, what happens to the motion of the particles and the pressure inside a sealed container?
▶️ Answer / Explanation
Step 1: According to the kinetic theory, the temperature of a gas is proportional to the average kinetic energy of its particles.
Step 2: As temperature increases, particles move faster and collide more frequently with the container walls.
Step 3: Since the container volume is fixed, the pressure increases due to these more frequent and forceful collisions.
Final Answer: Higher temperature → faster motion → increased pressure at constant volume.
Example:
The average kinetic energy of gas molecules at 300 K is \( \mathrm{6.21 \times 10^{-21}\ J} \). Calculate their average kinetic energy at 450 K.
▶️ Answer / Explanation
Step 1: Use proportionality: \( \mathrm{\dfrac{KE_2}{KE_1} = \dfrac{T_2}{T_1}} \).
Step 2: \( \mathrm{KE_2 = 6.21 \times 10^{-21} \times \dfrac{450}{300}} \).
Step 3: \( \mathrm{KE_2 = 9.32 \times 10^{-21}\ J} \).
Final Answer: The average kinetic energy at 450 K is \( \mathrm{9.32 \times 10^{-21}\ J} \).
Example :
Explain why a helium-filled balloon expands when placed in sunlight, using the kinetic theory of gases.
▶️ Answer / Explanation
Step 1: Sunlight increases the temperature of the gas inside the balloon.
Step 2: According to the kinetic theory, higher temperature increases the average kinetic energy of gas particles.
Step 3: Faster-moving helium atoms strike the inner surface of the balloon more frequently and with greater force.
Step 4: The increased internal pressure pushes outward, causing the balloon to expand until pressure inside and outside balance.
Final Answer: The balloon expands because heating increases the kinetic energy and pressure of the helium gas inside, consistent with the kinetic theory of gases.
