IB MYP 4-5 Chemistry -Percentage yield and atom economy- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Percentage yield and atom economy- Study Notes
Key Concepts
- Percentage Yield, Percentage Purity, and Atom Economy
Percentage Yield, Percentage Purity, and Atom Economy
Percentage Yield, Percentage Purity, and Atom Economy
In real chemical reactions, the amount of product obtained in practice is often less than the amount predicted theoretically. This is due to incomplete reactions, side reactions, or impurities. To evaluate efficiency and effectiveness, chemists use percentage yield, percentage purity, and atom economy.
Percentage Yield
The percentage yield compares the actual yield obtained from an experiment to the theoretical yield predicted from stoichiometric calculations.
\( \mathrm{Percentage\ Yield = \dfrac{Actual\ Yield}{Theoretical\ Yield} \times 100} \)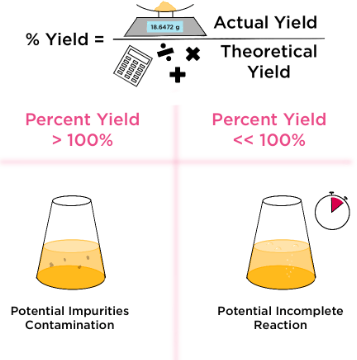
- Actual yield: The mass of product obtained from the experiment.
- Theoretical yield: The calculated maximum mass of product predicted from the balanced equation.
Key Idea: A yield less than 100% means some product was lost or the reaction was incomplete. A yield over 100% usually means impurities were present.
Percentage Purity
The percentage purity of a substance measures how much of the sample is the desired chemical, compared to the total sample mass.
\( \mathrm{Percentage\ Purity = \dfrac{Mass\ of\ Pure\ Substance}{Total\ Mass\ of\ Sample} \times 100} \)
Key Idea: Used when an impure reactant is used in a chemical reaction to determine how much of it is actually active material.
Atom Economy
The atom economy measures the efficiency of a chemical reaction in terms of how many of the atoms in the reactants are incorporated into the desired product.
\( \mathrm{Atom\ Economy = \dfrac{Molecular\ Mass\ of\ Desired\ Product}{Sum\ of\ Molecular\ Masses\ of\ All\ Products} \times 100} \)
- High atom economy → less waste, more sustainable reaction.
- Low atom economy → more by-products and waste.
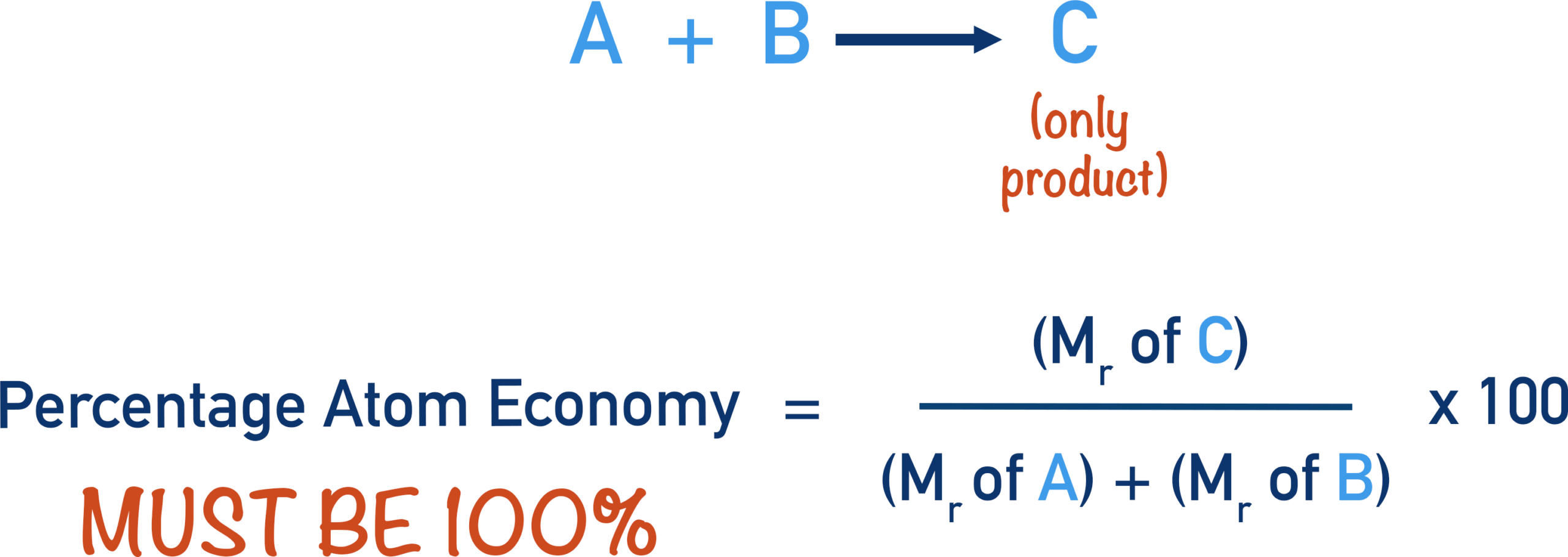
Yield, Purity, and Atom Economy
| Concept | Definition | Formula | Purpose |
|---|---|---|---|
| Percentage Yield | Compares actual yield to theoretical yield | \( \mathrm{\dfrac{Actual}{Theoretical} \times 100} \) | Measures reaction efficiency |
| Percentage Purity | Compares mass of pure substance to total sample | \( \mathrm{\dfrac{Pure}{Impure} \times 100} \) | Determines sample quality |
| Atom Economy | Compares useful product mass to total product mass | \( \mathrm{\dfrac{Mr\ of\ desired}{Total\ Mr\ of\ products} \times 100} \) | Measures reaction sustainability |
Example
Theoretical yield of magnesium oxide = 10.0 g, but only 8.5 g was obtained. Calculate the percentage yield.
▶️ Answer / Explanation
Step 1: \( \mathrm{Percentage\ Yield = \dfrac{8.5}{10.0} \times 100} \)
Step 2: \( \mathrm{= 85\%} \)
Final Answer: The percentage yield is 85%.
Example
A sample of impure calcium carbonate weighs 5 g and reacts to form 1.1 g of carbon dioxide. Calculate the percentage purity of the calcium carbonate. \( \mathrm{M(CaCO_3) = 100,\ M(CO_2) = 44} \)
▶️ Answer / Explanation
Step 1: Reaction: \( \mathrm{CaCO_3 \rightarrow CaO + CO_2} \)
Step 2: From equation: 1 mol \( \mathrm{CaCO_3} \) → 1 mol \( \mathrm{CO_2} \)
Step 3: \( \mathrm{n(CO_2) = \dfrac{1.1}{44} = 0.025\ mol} \)
Step 4: \( \mathrm{n(CaCO_3) = 0.025\ mol} \)
Step 5: \( \mathrm{m(CaCO_3) = nM = 0.025 \times 100 = 2.5\ g} \)
Step 6: \( \mathrm{Percentage\ Purity = \dfrac{2.5}{5.0} \times 100 = 50\%} \)
Final Answer: The sample is 50% pure.
Example
Ethene reacts with hydrogen to form ethane: \( \mathrm{C_2H_4 + H_2 \rightarrow C_2H_6} \)
Calculate the atom economy of this reaction.
▶️ Answer / Explanation
Step 1: \( \mathrm{Mr(C_2H_4) = 28,\ Mr(H_2) = 2,\ Mr(C_2H_6) = 30} \)
Step 2: \( \mathrm{Atom\ Economy = \dfrac{Mr\ of\ desired}{Total\ Mr\ of\ products} \times 100} \)
Step 3: \( \mathrm{= \dfrac{30}{30} \times 100 = 100\%} \)
Final Answer: The reaction has an atom economy of 100% (no waste).
