IB MYP 4-5 Chemistry -Periodic trends: reactivity, atomic radius, ionization- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Periodic trends: reactivity, atomic radius, ionization- Study Notes
Key Concepts
- Periodic Trends
Periodic Trends
Periodic Trends
Periodic trends are recurring patterns in the physical and chemical properties of elements as you move across a period (left → right) or down a group (top → bottom) in the periodic table. These trends arise because of systematic changes in atomic number, nuclear charge, and electron configuration.
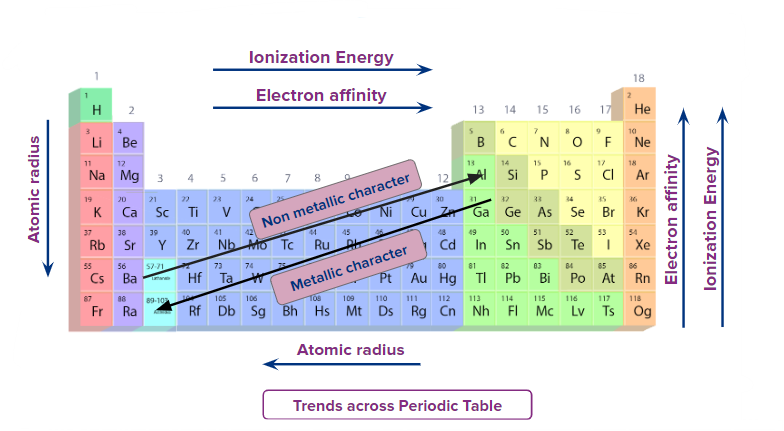
Understanding periodic trends helps predict element behavior, reactivity, and bonding tendencies.
Atomic Radius (Atomic Size)
The atomic radius is the distance from the nucleus to the outermost electron shell of an atom.
![]()
Trend:
- Across a period (→): Decreases.
- Down a group (↓): Increases.
Reason:
- Across → nuclear charge increases, pulling electrons closer to nucleus.
- Down → new electron shells are added → outer electrons farther away.
Exceptions:
- In Period 2, there is a slight increase in radius from Fluorine (F) to Neon (Ne) because Neon’s outer shell is completely filled and experiences more electron–electron repulsion.
- Transition metals show less change in atomic size across the period due to d-electron shielding (inner d-electrons reduce the pull of nucleus).
Trend summary: \( \mathrm{Size\ ↓\ across,\ ↑\ down} \)
Ionization Energy (IE)
Ionization energy is the minimum energy required to remove one electron from a neutral gaseous atom.
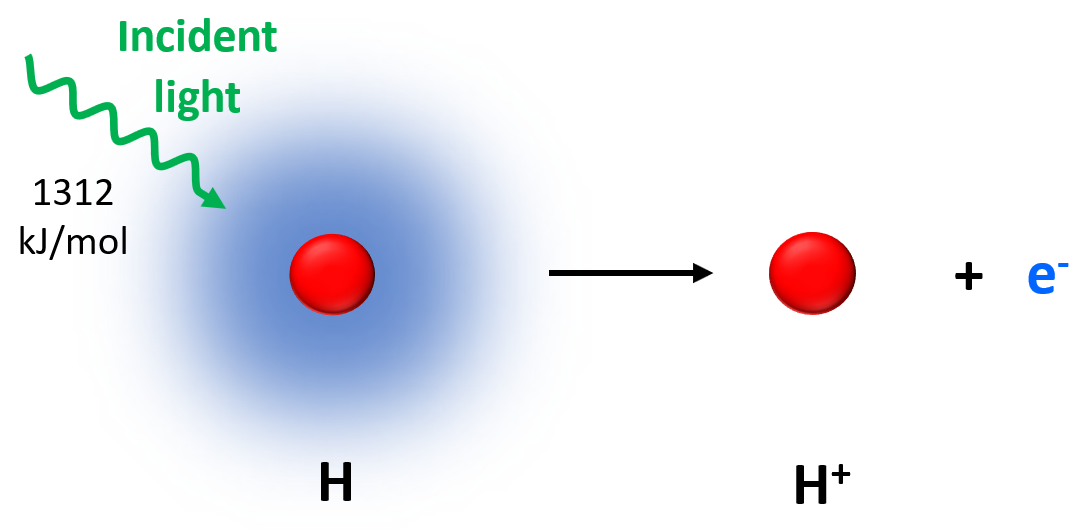
\( \mathrm{X(g) \rightarrow X^+(g) + e^-} \)
Trend:
- Across a period: Increases (atoms smaller, electrons tightly held).
- Down a group: Decreases (outer electrons farther from nucleus).
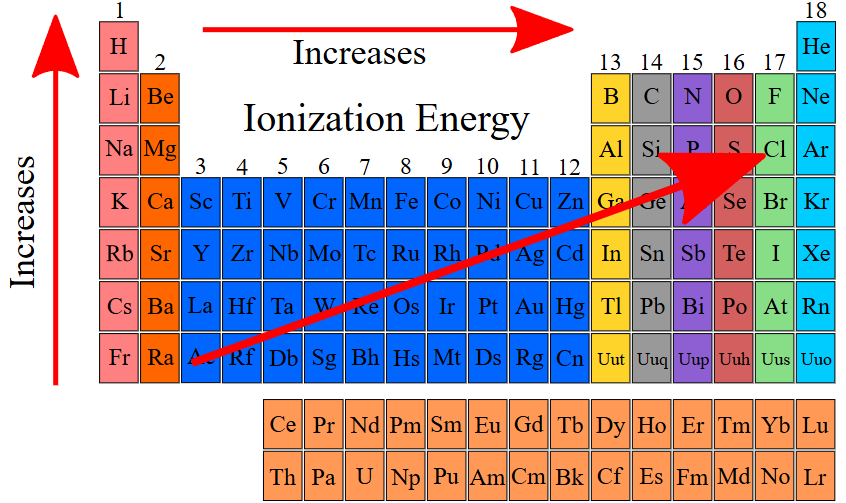
Exceptions (Important):
- In Period 2: \( \mathrm{Be} \) → \( \mathrm{B} \) (ionization energy decreases instead of increasing) because boron’s outer electron is in a new p-orbital, which is higher in energy and easier to remove.
- Similarly, \( \mathrm{N} \) → \( \mathrm{O} \) (ionization energy decreases) because in oxygen, two electrons pair up in one p-orbital, increasing repulsion and making it easier to remove one electron.
Reason: Ionization energy doesn’t always increase smoothly due to sub-shell arrangements and electron repulsion within orbitals.
Trend summary: \( \mathrm{IE\ ↑\ across,\ ↓\ down} \)
Electron Affinity (EA)
Electron affinity is the energy change when an atom gains an electron to form a negative ion.
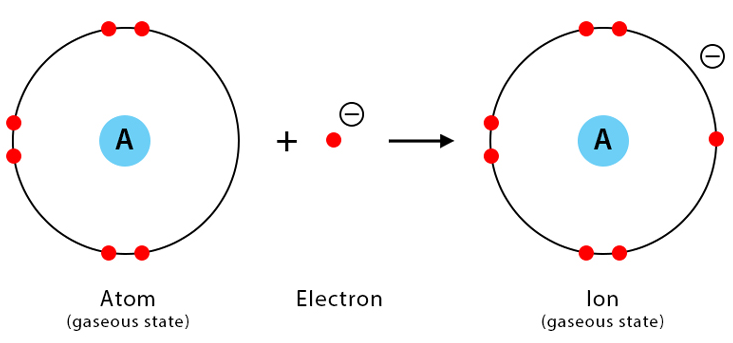
\( \mathrm{X(g) + e^- \rightarrow X^-(g)} \)
Trend:
- Across a period: Becomes more negative (greater attraction for added electrons).
- Down a group: Becomes less negative (weaker attraction).
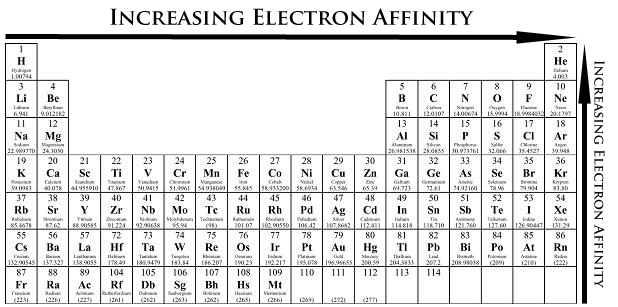
Exceptions:
- Group 2 (Be, Mg) and Group 18 (noble gases) have very low or positive electron affinity because their outer shells are already filled — no tendency to gain electrons.
- In Period 2, Fluorine has slightly lower (less negative) electron affinity than Chlorine, due to smaller size — extra electron faces more repulsion in the compact 2p shell.
Reason: Electron affinity depends on both nuclear charge and electron repulsion in outer shells.
Trend summary: \( \mathrm{EA\ ↑\ across,\ ↓\ down} \)
Electronegativity (EN)
Electronegativity is the tendency of an atom to attract electrons in a chemical bond.
Trend:
- Across a period: Increases (nuclear charge ↑, atomic size ↓).
- Down a group: Decreases (atomic size ↑, shielding ↑).
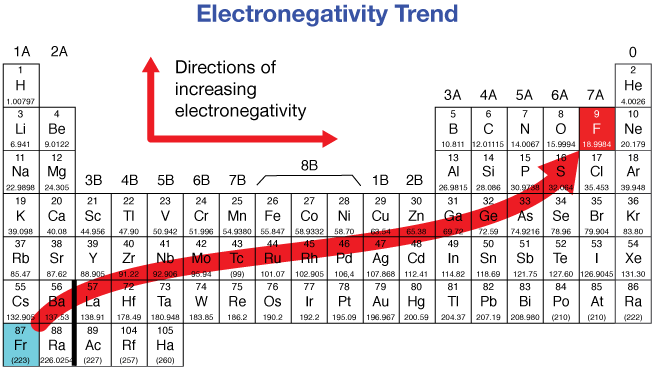
Exceptions:
- Transition metals have irregular electronegativity trends due to d-electron shielding and variable oxidation states.
- Group 18 noble gases have almost zero electronegativity because they do not usually form bonds.
Highest EN value: \( \mathrm{Fluorine\ (F) = 4.0} \)
Trend summary: \( \mathrm{EN\ ↑\ across,\ ↓\ down} \)
Metallic and Non-metallic Character
- Metallic character: Tendency of an atom to lose electrons and form positive ions.
- Non-metallic character: Tendency to gain or share electrons.
Trend:
- Across a period: Metallic character decreases; non-metallic character increases.
- Down a group: Metallic character increases.
Reason: Smaller atoms (right side) have stronger pull on electrons → non-metallic. Larger atoms (left side) lose electrons easily → metallic.
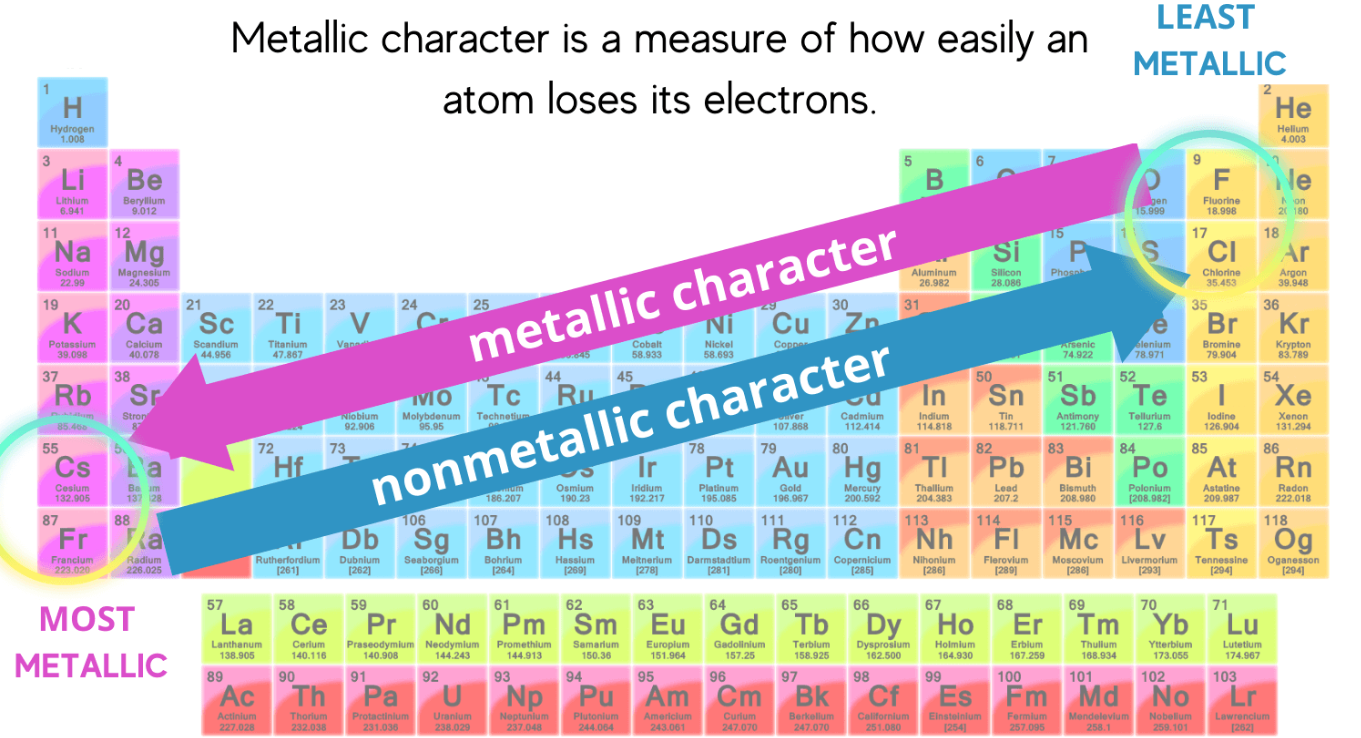
Exceptions:
- Hydrogen behaves as a non-metal, although placed in Group 1 (it forms both \( \mathrm{H^+} \) and \( \mathrm{H^-} \)).
- Metalloids (e.g., Si, As, Sb) show both metallic and non-metallic behavior — an exception to the clear divide.
Trend summary: \( \mathrm{Metallic\ ↓\ across,\ ↑\ down} \)
All Periodic Trends (with Exceptions)
| Property | Across a Period (→) | Down a Group (↓) | Key Exceptions |
|---|---|---|---|
| Atomic Radius | Decreases | Increases | Slight increase F → Ne (full shell repulsion) |
| Ionization Energy | Increases | Decreases | B < Be and O < N due to sub-shell and pairing effects |
| Electron Affinity | Becomes more negative | Less negative | Group 2 & 18 low EA; F < Cl due to repulsion |
| Electronegativity | Increases | Decreases | Irregular in transition metals; 0 in noble gases |
| Metallic Character | Decreases | Increases | Hydrogen and metalloids show dual behavior |
Example :
Why does atomic radius decrease across a period but increase down a group?
▶️ Answer / Explanation
Across a period: Nuclear charge increases while electron shells remain the same → stronger pull → smaller size.
Down a group: More shells → electrons farther → weaker pull → larger atoms.
Final Answer: Atomic radius decreases across and increases down due to nuclear attraction and added shells.
Example :
Explain why boron has a lower ionization energy than beryllium, even though it comes later in the period.
▶️ Answer / Explanation
Step 1: Be → outer electron in 2s orbital; B → outer electron in 2p orbital.
Step 2: 2p electron in B is farther and less tightly bound → easier to remove.
Final Answer: Ionization energy of B < Be because 2p orbital is higher in energy than 2s.
Example :
Fluorine has the highest electronegativity but a lower electron affinity than chlorine. Explain this apparent contradiction.
▶️ Answer / Explanation
Step 1: Fluorine’s small atomic size causes high electron–electron repulsion in the compact 2p orbital.
Step 2: Although it strongly attracts electrons (high EN), adding another electron releases slightly less energy (lower EA).
Step 3: Chlorine’s larger size allows the added electron to enter with less repulsion → higher EA value.
Final Answer: Fluorine’s high nuclear charge gives high electronegativity, but its small size reduces electron affinity due to repulsion effects.
