IB MYP 4-5 Chemistry -Polymers- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Polymers- Study Notes
Key Concepts
- Polymers (Addition and Condensation Polymers)
Polymers (Addition and Condensation Polymers)
Polymers (Addition and Condensation Polymers)
A polymer is a very large molecule made by joining together many small repeating units called monomers. The process of forming polymers from monomers is called polymerization.
Monomer → small molecule
Polymer → giant molecule made from many monomers linked in a chain
Key Terms
- Monomer: A small, reactive molecule that can join to other similar molecules to form a polymer. Example: Ethene (\( \mathrm{C_2H_4} \))
- Polymer: A long-chain molecule made of many repeating units. Example: Poly(ethene)
- Repeat unit: The specific arrangement of atoms that repeats again and again along the polymer chain.
Types of Polymerization
- Addition polymerization – Monomers add together with no small molecule produced.
Usually involves alkenes (C=C double bond). - Condensation polymerization – Monomers join together and small molecules such as water (H₂O) or HCl are released.
Usually involves monomers with two functional groups (e.g., –COOH, –OH, –NH₂).
Addition Polymers
In addition polymerization, many monomers with double bonds (usually alkenes) join together to form a long chain. The double bond in each monomer opens up and links with others.
General form: \( \mathrm{n\ CH_2=CHR \ \longrightarrow\ [-CH_2-CHR-]_n} \)
Example: Ethene → Poly(ethene)![]()
\( \mathrm{n\ CH_2=CH_2 \ \longrightarrow\ [-CH_2-CH_2-]_n} \)
- Monomer: Ethene (\( \mathrm{CH_2=CH_2} \))
- Polymer: Poly(ethene) (also called polythene or polyethylene)
- Uses: Plastic bags, bottles, toys
Example: Chloroethene → PVC![]()
\( \mathrm{n\ CH_2=CHCl \ \longrightarrow\ [-CH_2-CHCl-]_n} \)
- Monomer: Chloroethene (vinyl chloride)
- Polymer: PVC (polyvinyl chloride)
- Uses: Pipes, window frames, insulation
Properties of Addition Polymers:
- Usually non-biodegradable (they do not break down easily in nature).
- Often strong, flexible, waterproof.
- Poor conductors of electricity (good electrical insulators).
Condensation Polymers
In condensation polymerization, monomers with two functional groups react together to form long chains, and a small molecule like water or HCl is eliminated each time a link is formed.
Important: Each step forms a link AND releases a small molecule.
Example: Formation of a Polyester
React a diol (a molecule with two –OH groups) and a dicarboxylic acid (a molecule with two –COOH groups).
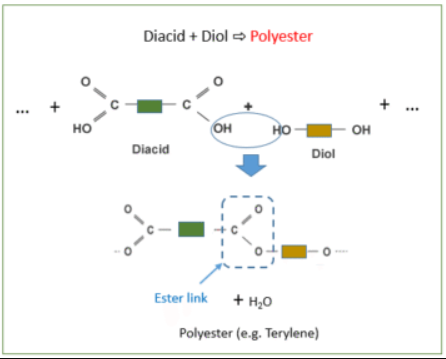
Diol: \( \mathrm{HO{-}R{-}OH} \)
Dicarboxylic acid: \( \mathrm{HOOC{-}R'{-}COOH} \)
They join by forming an ester link (-COO-) and release water.
\( \mathrm{-HO{-}R{-}OH + HOOC{-}R'{-}COOH- \rightarrow -[{-}R{-}OOC{-}R'{-}COO{-}]_n + H_2O} \)
- Polymer formed: Polyester
- Linkage type: Ester linkage (–COO–)
- Uses: Clothing fibres (e.g. PET), bottles, textiles
Example: Formation of a Polyamide (like Nylon)
React a diamine (two –NH₂ groups) with a dicarboxylic acid (two –COOH groups).
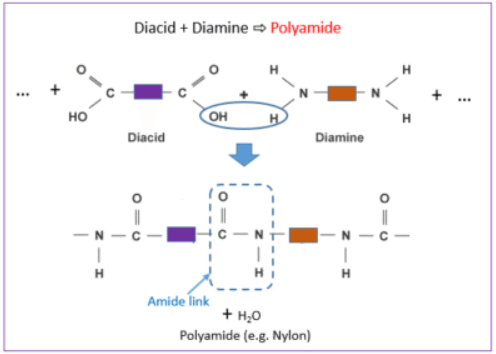
Diamine: \( \mathrm{H_2N{-}R{-}NH_2} \)
Dicarboxylic acid: \( \mathrm{HOOC{-}R'{-}COOH} \)
They join by forming an amide link (–CONH–) and release water.
\( \mathrm{-H_2N{-}R{-}NH_2 + HOOC{-}R'{-}COOH- \rightarrow -[{-}R{-}NHCO{-}R'{-}CONH{-}]_n + H_2O} \)
- Polymer formed: Polyamide (e.g. Nylon)
- Linkage type: Amide link (–CONH–)
- Uses: Ropes, fishing line, parachutes, engineering plastics
Addition vs. Condensation Polymers — Summary Table
| Feature | Addition Polymer | Condensation Polymer |
|---|---|---|
| Monomer type | Alkene (C=C) | Monomers with 2 functional groups (e.g. –COOH, –OH, –NH₂) |
| By-product released? | No | Yes (e.g. H₂O, HCl) |
| Example polymer | Poly(ethene), PVC | Polyester, Nylon |
| Typical uses | Plastic bags, packaging | Clothing fibres, ropes |
| Biodegradability | Usually not biodegradable | Some can be biodegradable (especially certain polyesters) |
Environmental Impact of Polymers
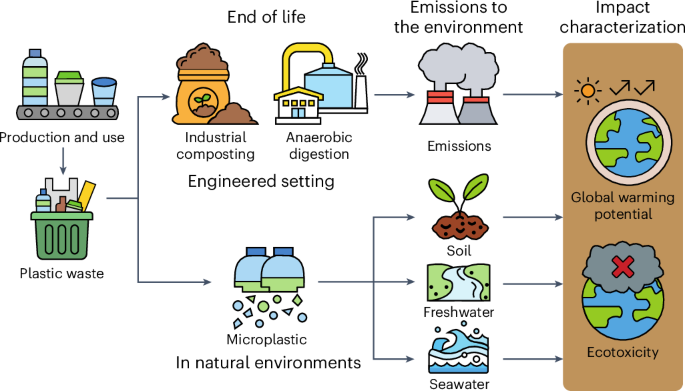
- Non-biodegradability: Many addition polymers (like poly(ethene)) do not break down easily. They persist in landfills and oceans for many years.
- Microplastics: Plastics can break into tiny particles that enter food chains and harm wildlife.
- Recycling: Some plastics can be melted and reshaped (thermoplastics). Others are harder to recycle.
- Incineration issues: Burning plastics can release toxic gases, e.g. burning PVC can release hydrogen chloride gas (\( \mathrm{HCl} \)).
- Bioplastics and biodegradable polymers: Research is moving toward polymers that can break down naturally to reduce pollution.
Everyday Uses of Important Polymers
| Polymer | Made From | Type | Uses |
|---|---|---|---|
| Poly(ethene) | Ethene | Addition polymer | Carrier bags, bottles |
| PVC | Chloroethene | Addition polymer | Pipes, window frames |
| Polyester | Diol + Dicarboxylic acid | Condensation polymer | Clothing fibres, bottles |
| Nylon (polyamide) | Diamine + Dicarboxylic acid | Condensation polymer | Ropes, parachutes, fishing lines |
Example
Write the polymerization equation for propene and name the polymer formed.
▶️ Answer / Explanation
Step 1: Propene monomer is \( \mathrm{CH_3CH=CH_2} \).
Step 2: During addition polymerization, the double bonds open and link.
\( \mathrm{n\ CH_3CH=CH_2 \ \longrightarrow\ [-CH_2-CH(CH_3)-]_n} \)
Final Answer: The polymer is called poly(propene) (also known as polypropylene).
Example
How can you tell, by looking at a polymer’s repeat unit, whether it formed by addition polymerization or condensation polymerization?
▶️ Answer / Explanation
Step 1: If the repeat unit looks like it came from a single monomer with a C=C double bond that opened up → it is an addition polymer.
Step 2: If the repeat unit contains linking groups such as –COO– (ester link) or –CONH– (amide link), and could have come from two different monomers with functional groups → it is a condensation polymer.
Final Answer: Presence of linking groups like –COO– or –CONH– is a sign of condensation polymerization.
Example
Discuss one environmental problem caused by addition polymers and suggest one realistic solution.
▶️ Answer / Explanation
Step 1: Problem: Many addition polymers such as poly(ethene) and PVC are not biodegradable. They accumulate in landfills and oceans, where they can harm animals and break down into microplastics.
Step 2: Solution: Improve plastic recycling and design polymers that can be depolymerized (chemically broken back into monomers) for reuse, or replace some single-use plastics with biodegradable polymers and reusable materials.
Final Answer: Non-biodegradable plastics cause long-term pollution, and controlled recycling plus biodegradable alternatives helps reduce this impact.
