IB MYP 4-5 Chemistry -Pure and impure substances- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Pure and impure substances- Study Notes
Key Concepts
- Pure and Impure Substances
- Mixtures and Their Types (Homogeneous & Heterogeneous)
Pure and Impure Substances
Pure and Impure Substances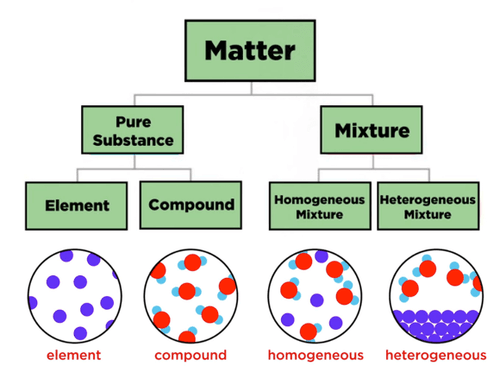
All matter around us is made up of substances. A pure substance contains only one type of particle, while an impure substance (or mixture) contains two or more different kinds of particles physically combined.
Pure Substances
A pure substance is a material that contains only one kind of element or compound and has a fixed composition throughout.

- Every portion of a pure substance has the same properties and composition.
- It has a definite melting point and boiling point.
- Its composition cannot be separated by physical methods (like filtration or distillation).
Examples:
- Elements — oxygen (\( \mathrm{O_2} \)), gold (\( \mathrm{Au} \)), iron (\( \mathrm{Fe} \))
- Compounds — water (\( \mathrm{H_2O} \)), carbon dioxide (\( \mathrm{CO_2} \)), sodium chloride (\( \mathrm{NaCl} \))
Impure Substances (Mixtures)
An impure substance is made up of two or more different elements or compounds that are physically combined in any proportion.

- Its components retain their individual properties.
- The mixture’s composition can vary.
- It does not have a fixed melting or boiling point — these occur over a range.
- The components can be separated by physical methods such as filtration, evaporation, distillation, or chromatography.
Examples:
- Air (a mixture of gases like \( \mathrm{N_2} \), \( \mathrm{O_2} \), \( \mathrm{CO_2} \))
- Sea water (water + salts)
- Alloys (brass = copper + zinc)
- Soil (sand + minerals + organic matter)
Comparison Between Pure and Impure Substances
| Property | Pure Substance | Impure Substance (Mixture) |
|---|---|---|
| Composition | Fixed composition of one type of particle | Variable composition of different substances |
| Melting / Boiling Point | Sharp and definite | Occurs over a range of temperatures |
| Separation | Cannot be separated by physical methods | Can be separated by physical methods |
| Properties | Same throughout; constant physical and chemical properties | Properties vary depending on proportion of components |
| Examples | Distilled water, pure oxygen, diamond | Sea water, air, milk, alloys |
Importance of Purity
Purity is essential in many applications, especially in medicine, food, and chemistry: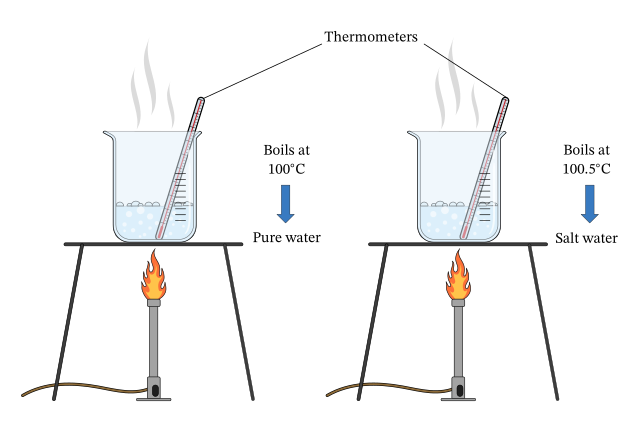
- Pharmaceutical drugs must be chemically pure for safe dosage.
- In industry, impurities can alter melting points and cause equipment corrosion.
- Pure chemicals ensure consistent reactions and predictable results in laboratories.
Checking Purity
Purity of a substance can be checked by observing its melting or boiling point:
- A pure substance melts/boils sharply at a fixed temperature.
- An impure substance melts/boils over a range and often at a slightly different temperature from the pure substance.
Example: Pure water boils at exactly \( \mathrm{100^\circ C} \) at 1 atm pressure. If salts or impurities are dissolved in it, the boiling point rises above \( \mathrm{100^\circ C} \).
Example:
Why is distilled water considered pure while tap water is not?
▶️ Answer / Explanation
Step 1: Distilled water contains only \( \mathrm{H_2O} \) molecules, with all dissolved salts and impurities removed.
Step 2: Tap water contains dissolved minerals, gases, and microorganisms.
Final Answer: Distilled water is pure because it has a fixed composition and definite boiling point, while tap water is a mixture of water and dissolved impurities, making it impure.
Example :
A sample of metal melts over a range of temperatures from 610°C to 640°C. What does this indicate about the sample?
▶️ Answer / Explanation
Step 1: A pure substance has a sharp melting point.
Step 2: Melting over a range shows the presence of different substances (impurities or alloys).
Final Answer: The sample is impure because it melts over a range of temperatures instead of at a single, definite temperature.
Example :
Explain why the presence of impurities in a metal affects its physical properties such as melting point and electrical conductivity.
▶️ Answer / Explanation
Step 1: Impurities disrupt the regular arrangement of atoms in the pure metal.
Step 2: This makes it more difficult for atoms to move freely and requires more or less energy for melting, thus altering the melting point.
Step 3: Impurities also scatter moving electrons, reducing the metal’s ability to conduct electricity efficiently.
Final Answer: Impurities lower the purity and alter properties by disturbing atomic structure, changing both melting point and electrical conductivity.
Mixtures and Their Types (Homogeneous & Heterogeneous)
Mixtures and Their Types (Homogeneous & Heterogeneous)
A mixture is a combination of two or more substances that are physically combined in any proportion and can be separated by physical methods. In a mixture, the individual substances retain their original chemical identities and properties.
Characteristics of Mixtures
- Components are not chemically combined.
- They can be present in any ratio.
- Each component retains its own physical and chemical properties.
- Can be separated by physical methods such as filtration, evaporation, distillation, or chromatography.
- Mixtures usually do not have fixed melting or boiling points — they occur over a range.
Types of Mixtures
Homogeneous Mixtures
A homogeneous mixture has a uniform composition throughout; its components are evenly distributed, and individual substances cannot be distinguished by the naked eye.

- They consist of only one visible phase.
- Particle size is extremely small (molecular or ionic level).
- They are also known as solutions.
Examples:
- Salt dissolved in water
- Air (mixture of gases)
- Sugar solution
- Alloys like brass (copper + zinc)
Heterogeneous Mixtures
A heterogeneous mixture has a non-uniform composition, and the different components can be seen or separated easily.

- They consist of two or more visible phases.
- Particles are large enough to be seen or filtered.
- Different regions of the mixture may have different compositions.
Examples:
- Sand and water
- Oil and water
- Fruit salad
- Soil (sand + humus + stones)
Comparison Table: Homogeneous vs. Heterogeneous Mixtures*
| Property | Homogeneous Mixture | Heterogeneous Mixture |
|---|---|---|
| Uniformity | Uniform composition throughout | Non-uniform composition |
| Number of Phases | Single phase | Two or more distinct phases |
| Visibility of Components | Components not visible | Components easily visible |
| Particle Size | Very small (molecular/ionic level) | Larger, can be seen or filtered |
| Examples | Air, salt solution, brass | Oil and water, sand and iron, fruit salad |
Subtypes of Mixtures
Mixtures can also be grouped according to the state of matter of their components:
| Type | Example | Nature |
|---|---|---|
| Solid + Solid | Alloy (brass = Cu + Zn) | Homogeneous |
| Solid + Liquid | Salt in water | Homogeneous |
| Liquid + Liquid | Alcohol and water | Homogeneous |
| Liquid + Gas | Carbonated drink (CO₂ + water) | Heterogeneous if gas bubbles visible |
| Gas + Gas | Air (N₂ + O₂ + CO₂) | Homogeneous |
Example :
Is air a homogeneous or heterogeneous mixture? Justify your answer.
▶️ Answer / Explanation
Step 1: Air is made up of gases like \( \mathrm{N_2} \), \( \mathrm{O_2} \), \( \mathrm{CO_2} \), and others mixed uniformly.
Step 2: Each component is evenly distributed, and the mixture appears as a single phase.
Final Answer: Air is a homogeneous mixture because its composition is uniform throughout.
Example :
Why is a mixture of oil and water considered heterogeneous even after shaking?
▶️ Answer / Explanation
Step 1: Oil and water do not dissolve in each other — they are immiscible liquids.
Step 2: Even when shaken, small oil droplets remain suspended temporarily but soon separate into two distinct layers.
Final Answer: The mixture is heterogeneous because it has two visible phases (oil and water) and a non-uniform composition.
Example :
Explain how an alloy like brass can be a homogeneous mixture, even though it contains more than one element.
▶️ Answer / Explanation
Step 1: Brass is made by melting copper and zinc together, allowing atoms to mix uniformly in the molten state.
Step 2: Once solidified, the mixture appears as a single phase with uniform composition.
Step 3: No distinct layers or visible differences can be seen between the metals.
Final Answer: Brass is a homogeneous mixture because its components are uniformly distributed at the atomic level, forming one visible phase.
