IB MYP 4-5 Chemistry -Reacting mass calculations and limiting reagents- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Reacting mass calculations and limiting reagents- Study Notes
Key Concepts
- Reacting Mass Calculations and Limiting Reagents
Reacting Mass Calculations and Limiting Reagents
Reacting Mass Calculations and Limiting Reagents
Reacting mass calculations are used to determine the mass of reactants and products in a chemical reaction using mole relationships derived from the balanced chemical equation.
Key Idea: Balanced equations show the mole ratio of substances. By converting masses into moles, we can find how much of one substance reacts with or produces another.
Steps for Reacting Mass Calculations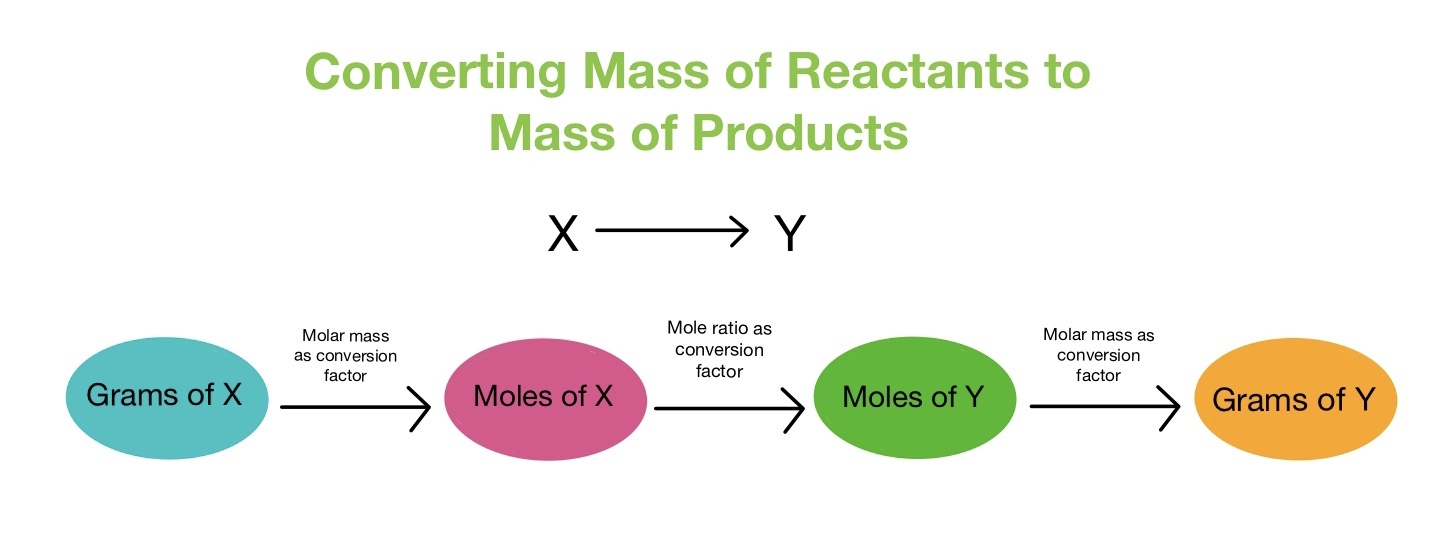
- Write a balanced chemical equation.
- Find the molar mass (Mr) of all relevant substances.
- Convert the known mass to moles using \( \mathrm{n = \dfrac{m}{M}} \).
- Use the mole ratio from the equation to find moles of the unknown substance.
- Convert the moles of the unknown back to mass using \( \mathrm{m = n \times M} \).
Formula Summary:
\( \mathrm{n = \dfrac{m}{M}} \ \text{and} \ \mathrm{m = n \times M} \)
Example of a Balanced Equation Showing Mole Ratio
\( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \)
- Mole ratio of \( \mathrm{H_2 : O_2 : H_2O = 2 : 1 : 2} \)
- This means 2 moles of hydrogen react with 1 mole of oxygen to form 2 moles of water.
The Limiting Reagent Concept
When reactants are not mixed in their exact mole ratio, one reactant will be completely used up first. This reactant is called the limiting reagent because it limits the amount of product formed.
![]()
- Limiting reagent: Reactant that is fully consumed first.
- Excess reagent: Reactant that remains unreacted after the reaction stops.
Key Idea: The limiting reagent determines the maximum yield of products possible in a reaction.
Reacting Mass and Limiting Reagent Concepts
| Concept | Description | Key Formula |
|---|---|---|
| Reacting Mass | Calculating product/reactant masses using mole ratios | \( \mathrm{m = nM} \) |
| Limiting Reagent | Substance that determines the maximum amount of product | Compare available moles to required mole ratio |
| Excess Reagent | Substance remaining after reaction completes | Find leftover moles after limiting reagent consumed |
Example
Hydrogen reacts with oxygen to form water: \( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \). Calculate the mass of water formed when 4 g of hydrogen reacts completely.
▶️ Answer / Explanation
Step 1: \( \mathrm{M(H_2) = 2\ g/mol,\ M(H_2O) = 18\ g/mol} \)
Step 2: \( \mathrm{n(H_2) = \dfrac{4}{2} = 2\ mol} \)
Step 3: From equation, \( \mathrm{2\ mol\ H_2 \rightarrow 2\ mol\ H_2O} \)
Step 4: \( \mathrm{n(H_2O) = 2\ mol} \)
Step 5: \( \mathrm{m = nM = 2 \times 18 = 36\ g} \)
Final Answer: 36 g of water is formed.
Example
Magnesium reacts with hydrochloric acid according to: \( \mathrm{Mg + 2HCl \rightarrow MgCl_2 + H_2} \). Find the mass of hydrogen produced when 6.08 g of magnesium reacts completely with excess acid. \( \mathrm{M(Mg) = 24.3,\ M(H_2) = 2.0} \)
▶️ Answer / Explanation
Step 1: \( \mathrm{n(Mg) = \dfrac{6.08}{24.3} = 0.25\ mol} \)
Step 2: From equation, 1 mol Mg → 1 mol \( \mathrm{H_2} \)
Step 3: \( \mathrm{n(H_2) = 0.25\ mol} \)
Step 4: \( \mathrm{m = nM = 0.25 \times 2 = 0.5\ g} \)
Final Answer: \( \mathrm{0.5\ g} \) of hydrogen is produced.
Example
Given the reaction: \( \mathrm{2Al + 3Cl_2 \rightarrow 2AlCl_3} \). 13.5 g of aluminum reacts with 40 g of chlorine gas. Find:
- (a) The limiting reagent
- (b) The mass of aluminum chloride formed
\( \mathrm{M(Al) = 27,\ M(Cl_2) = 71,\ M(AlCl_3) = 133.5} \)
▶️ Answer / Explanation
Step 1: \( \mathrm{n(Al) = \dfrac{13.5}{27} = 0.5\ mol} \)
Step 2: \( \mathrm{n(Cl_2) = \dfrac{40}{71} = 0.563\ mol} \)
Step 3: From equation: \( \mathrm{2Al : 3Cl_2} \Rightarrow 1 : 1.5} \)
For 0.5 mol Al, required \( \mathrm{Cl_2 = 0.5 \times 1.5 = 0.75\ mol} \)
But available \( \mathrm{Cl_2 = 0.563\ mol} \)
→ Limiting reagent = \( \mathrm{Cl_2} \)
Step 4: From the equation, \( \mathrm{3Cl_2 \rightarrow 2AlCl_3} \)
\( \mathrm{n(AlCl_3) = \dfrac{2}{3} \times n(Cl_2) = \dfrac{2}{3} \times 0.563 = 0.375\ mol} \)
Step 5: \( \mathrm{m(AlCl_3) = nM = 0.375 \times 133.5 = 50.1\ g} \)
Final Answers:
- (a) Limiting reagent = \( \mathrm{Cl_2} \)
- (b) Mass of \( \mathrm{AlCl_3} = 50.1\ g \)
