IB MYP 4-5 Chemistry -Reactivity series of metals- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Reactivity series of metals- Study Notes
Key Concepts
- Reactivity Series of Metals
Reactivity Series of Metals
Reactivity Series of Metals
The reactivity series is a list of metals arranged in order of their ability to lose electrons to form positive ions \( \mathrm{(M^{n+})} \). The most reactive metals are placed at the top, and the least reactive at the bottom.
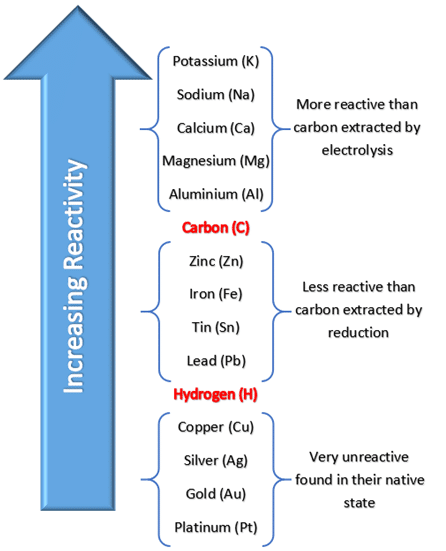
The reactivity of a metal depends on how easily it can lose electrons to form positive ions. Metals that lose electrons more readily are more reactive.
Reactivity Series (from Most to Least Reactive)
| Position | Metal | Symbol | Typical Reaction Observation |
|---|---|---|---|
| 1 | Potassium | K | Very vigorous reaction with water — bursts into flames |
| 2 | Sodium | Na | Vigorous reaction with water — melts and moves on surface |
| 3 | Calcium | Ca | Reacts steadily with water to form bubbles |
| 4 | Magnesium | Mg | Burns with a bright white flame in air; reacts slowly with cold water |
| 5 | Aluminium | Al | Reacts only when oxide layer is removed; strong reducing agent |
| 6 | Zinc | Zn | Reacts moderately with acid; forms hydrogen gas |
| 7 | Iron | Fe | Slowly reacts with acids; rusts in moist air |
| 8 | Tin | Sn | Mild reaction with strong acids only |
| 9 | Lead | Pb | Reacts with hot concentrated acids |
| 10 | Hydrogen | H | Reference (non-metal) |
| 11 | Copper | Cu | Does not react with dilute acids |
| 12 | Silver | Ag | Very low reactivity; tarnishes slowly in air |
| 13 | Gold | Au | Unaffected by air, water, or acids |
Explanation of Reactivity
Metals react by losing electrons to form cations: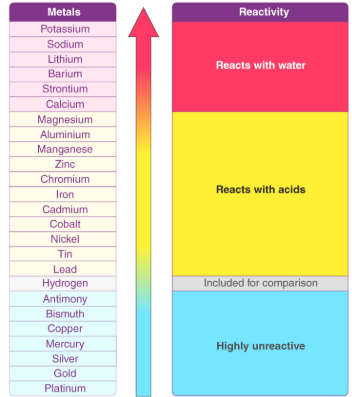
\( \mathrm{M \rightarrow M^{n+} + ne^-} \)
The ease of electron loss determines reactivity:
- Highly reactive metals (e.g., K, Na, Ca) lose electrons easily form strong ionic bonds.
- Less reactive metals (e.g., Cu, Ag, Au) hold onto their electrons tightly resist oxidation.
Reactions Used to Determine Metal Reactivity
Reactivity is compared using reactions with:
- Water: Highly reactive metals (K, Na, Ca) react with water to form hydrogen gas.
- Acids: Moderately reactive metals (Mg, Zn, Fe) react with acids to form hydrogen gas.
- Metal salts (displacement): More reactive metal displaces a less reactive metal from its salt solution.
Displacement Example:
\( \mathrm{Zn + CuSO_4 \rightarrow ZnSO_4 + Cu} \)
Zinc displaces copper because it is more reactive.
Reactions with Water and Acids
| Metal | Reaction with Cold Water | Reaction with Acid |
|---|---|---|
| Potassium, Sodium, Calcium | Vigorous, forms hydrogen gas | Too violent to test |
| Magnesium, Zinc, Iron | Little or no reaction with cold water | Steady reaction with hydrogen gas evolution |
| Copper, Silver, Gold | No reaction | Do not react with dilute acids |
Example
What will happen if a piece of zinc is placed in copper(II) sulfate solution?
▶️ Answer / Explanation
Step 1: Zinc is above copper in the reactivity series.
Step 2: Zinc displaces copper from its compound:
\( \mathrm{Zn + CuSO_4 \rightarrow ZnSO_4 + Cu} \)
Final Answer: Copper is deposited and the blue color of solution fades.
Example
Explain why aluminium appears unreactive even though it is high in the reactivity series.
▶️ Answer / Explanation
Step 1: Aluminium reacts quickly with oxygen in air forming a thin layer of aluminium oxide.
Step 2: This oxide layer is tough and prevents further reaction.
Final Answer: Aluminium is actually very reactive, but its protective oxide layer makes it appear unreactive.
Example
A student adds iron filings to lead(II) nitrate solution. Will a reaction occur? Explain using the reactivity series.
▶️ Answer / Explanation
Step 1: Iron is above lead in the reactivity series.
Step 2: Iron displaces lead from its salt solution:
\( \mathrm{Fe + Pb(NO_3)_2 \rightarrow Fe(NO_3)_2 + Pb} \)
Step 3: Gray lead metal will form at the bottom of the beaker.
Final Answer: Yes — iron displaces lead, confirming that iron is more reactive than lead.
