IB MYP 4-5 Chemistry -Reversible reactions and equilibrium (qualitative)- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Reversible reactions and equilibrium (qualitative)- Study Notes
Key Concepts
- Reversible Reactions and Equilibrium (Qualitative)
- Le Chatelier’s Principle (Qualitative Explanation and Applications)
Reversible Reactions and Equilibrium (Qualitative)
Reversible Reactions and Equilibrium (Qualitative)
A reversible reaction is a chemical reaction that can proceed in both the forward and reverse directions. The products formed can react again to produce the original reactants.
\( \mathrm{A + B \rightleftharpoons C + D} \)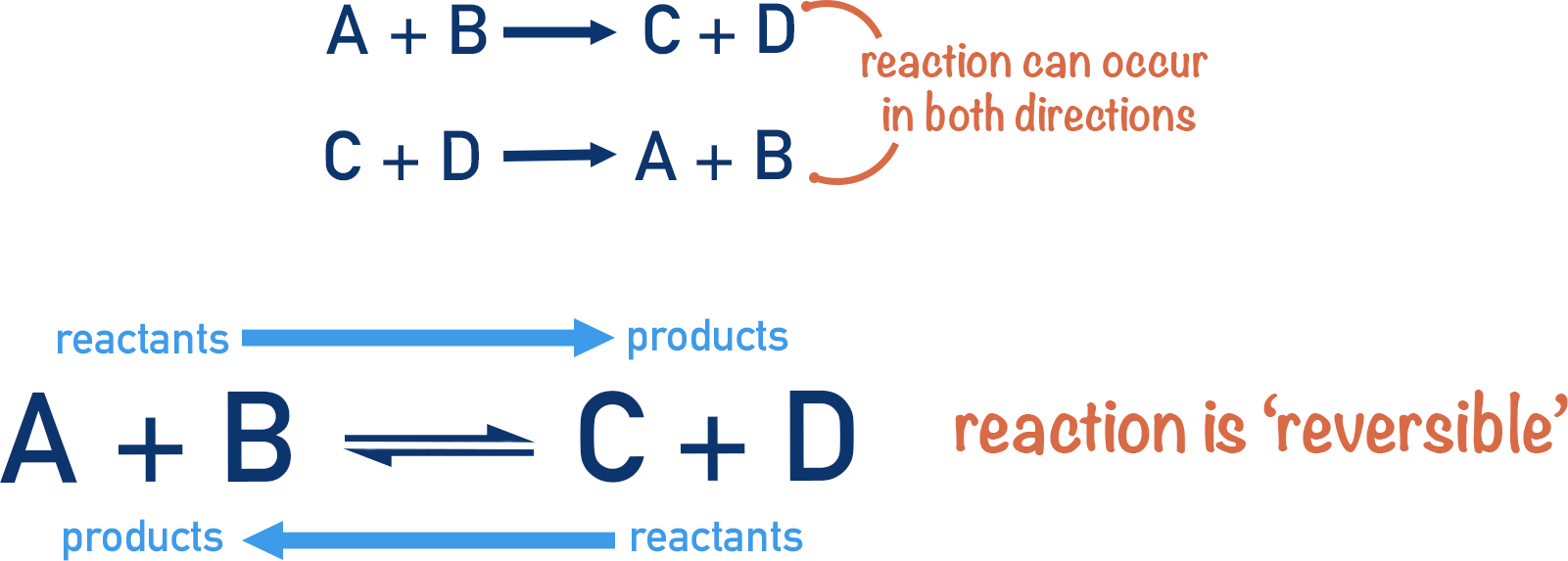
- The forward reaction: \( \mathrm{A + B \rightarrow C + D} \)
- The reverse reaction: \( \mathrm{C + D \rightarrow A + B} \)
Reversible reactions never go to completion instead, they reach a point of dynamic equilibrium.
Dynamic Equilibrium
Dynamic equilibrium is the state in a reversible reaction when the rate of the forward reaction equals the rate of the reverse reaction, and the concentrations of reactants and products remain constant.
![]()
- The reaction continues both ways, but there is no net change in amounts of reactants and products.
- The system is dynamic (particles still react), not static.
Conditions for Equilibrium:![]()
- The system must be closed — no substances can enter or leave.
- Occurs when rates of forward and reverse reactions are equal.
- Concentrations remain constant, though reactions continue.
Representing Reversible Reactions
The symbol \( \mathrm{\rightleftharpoons} \) shows a reversible reaction.
Examples:
- \( \mathrm{N_2 + 3H_2 \rightleftharpoons 2NH_3} \) (Haber process)
- \( \mathrm{2SO_2 + O_2 \rightleftharpoons 2SO_3} \) (Contact process)
- \( \mathrm{CuSO_4 \cdot 5H_2O \rightleftharpoons CuSO_4 + 5H_2O} \) (hydrated ↔ anhydrous copper sulfate)
Characteristics of Dynamic Equilibrium
| Feature | Description |
|---|---|
| Closed System | No reactants or products can escape or enter. |
| Rate Equality | Rate of forward reaction = rate of reverse reaction. |
| Constant Concentrations | Amounts of reactants and products remain constant over time. |
| Dynamic Nature | Both forward and reverse reactions continue to occur simultaneously. |
Examples of Reversible Reactions in Daily Life
| Reaction | Forward Reaction | Reverse Reaction | Observation |
|---|---|---|---|
| Haber process | \( \mathrm{N_2 + 3H_2 \rightarrow 2NH_3} \) | \( \mathrm{2NH_3 \rightarrow N_2 + 3H_2} \) | Ammonia production and decomposition balance. |
| Hydrated Copper Sulfate | \( \mathrm{CuSO_4 + 5H_2O \rightarrow CuSO_4 \cdot 5H_2O} \) | \( \mathrm{CuSO_4 \cdot 5H_2O \rightarrow CuSO_4 + 5H_2O} \) | Blue ↔ White (with heat and water). |
| Nitrogen Dioxide ↔ Dinitrogen Tetroxide | \( \mathrm{2NO_2 \rightarrow N_2O_4} \) | \( \mathrm{N_2O_4 \rightarrow 2NO_2} \) | Brown ↔ Colourless (temperature dependent). |
Example
Explain what is meant by a reversible reaction using the dehydration of hydrated copper(II) sulfate.
▶️ Answer / Explanation
Step 1: Heating blue hydrated copper(II) sulfate crystals removes water.
Step 2: The white anhydrous copper(II) sulfate can reabsorb water to become blue again.
Final Answer: This shows a reversible reaction — \( \mathrm{CuSO_4 \cdot 5H_2O \rightleftharpoons CuSO_4 + 5H_2O} \).
Example
In the reaction \( \mathrm{2NO_2 \rightleftharpoons N_2O_4} \), what will happen if temperature is increased?
▶️ Answer / Explanation
Step 1: The forward reaction (formation of \( \mathrm{N_2O_4} \)) is exothermic.
Step 2: Increasing temperature favours the endothermic (reverse) reaction.
Step 3: The equilibrium shifts left, producing more brown \( \mathrm{NO_2} \).
Final Answer: The mixture becomes darker brown because more \( \mathrm{NO_2} \) forms.
Example
Ammonia is produced by the Haber process: \( \mathrm{N_2 + 3H_2 \rightleftharpoons 2NH_3 + Energy} \) Predict the direction of shift when:
- Pressure is increased
- Temperature is increased
▶️ Answer / Explanation
Step 1: Increasing pressure favours the side with fewer gas molecules.
Step 2: Left side has 4 mol of gas (1N₂ + 3H₂), right side has 2 mol (2NH₃).
Step 3: So, increasing pressure shifts equilibrium to the right → more ammonia formed.
Step 4: Increasing temperature favours endothermic (reverse) reaction.
Final Answer: • Higher pressure → more ammonia. • Higher temperature → less ammonia (shifts left).
Le Chatelier’s Principle (Qualitative Explanation and Applications)
Le Chatelier’s Principle (Qualitative Explanation and Applications)
Le Chatelier’s Principle states that: “When a system at equilibrium is subjected to a change in temperature, pressure, or concentration, the equilibrium position shifts in the direction that tends to oppose the change and restore a new equilibrium.”
![]()
Understanding the Principle
- If the equilibrium is disturbed, the reaction will shift (forward or backward) to reduce the effect of that disturbance.
- The total energy of the system remains constant once the new equilibrium is established.
- The position of equilibrium changes, but the equilibrium constant \( \mathrm{K_{eq}} \) remains the same (for a fixed temperature).
![]()
Effect of Changing Concentration
Rule: If the concentration of a reactant or product changes, the equilibrium shifts to oppose the change by using up or producing that substance.
- Increase in reactant → shifts right (forward reaction favoured).
- Increase in product → shifts left (reverse reaction favoured).
- Decrease → shifts toward the side that replaces the substance removed.
Example: \( \mathrm{N_2 + 3H_2 \rightleftharpoons 2NH_3} \) If more \( \mathrm{N_2} \) is added → equilibrium shifts right → more \( \mathrm{NH_3} \) forms.
Effect of Changing Pressure
Rule: Pressure changes affect only gaseous equilibria.
- Increase in pressure → equilibrium shifts toward the side with fewer gas molecules.
- Decrease in pressure → shifts toward the side with more gas molecules.
- If number of gas molecules is equal on both sides → pressure change has no effect.
Example: \( \mathrm{N_2 + 3H_2 \rightleftharpoons 2NH_3} \) Left side has 4 mol gas, right has 2 mol → increasing pressure shifts right → more ammonia.
Effect of Changing Temperature
Rule: Changing temperature shifts equilibrium toward the endothermic or exothermic direction depending on whether heat is added or removed.
- Increase in temperature → equilibrium shifts in endothermic direction (absorbs heat).
- Decrease in temperature → equilibrium shifts in exothermic direction (releases heat).
Example: \( \mathrm{N_2 + 3H_2 \rightleftharpoons 2NH_3 + Energy} \) Raising temperature → favours reverse (endothermic) reaction → less ammonia formed.
Effect of a Catalyst
Rule: A catalyst does not shift the position of equilibrium; it only helps the system reach equilibrium faster by lowering the activation energy for both forward and reverse reactions equally.
Summary Table — Effect of Different Changes on Equilibrium
| Change Applied | System Response | Direction of Shift |
|---|---|---|
| Increase in Reactant Concentration | Uses up added reactant | Right (toward products) |
| Increase in Product Concentration | Uses up added product | Left (toward reactants) |
| Increase in Pressure | Reduces total gas molecules | Toward side with fewer gas moles |
| Increase in Temperature | Absorbs excess heat | Endothermic direction |
| Adding a Catalyst | No shift; equilibrium reached faster | No change |
Industrial Application — Haber Process (Example)
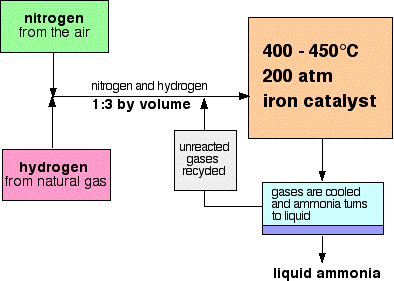
Reaction: \( \mathrm{N_2(g) + 3H_2(g) \rightleftharpoons 2NH_3(g) + Energy} \)
- High Pressure (≈ 200 atm): favours forward reaction → more ammonia (fewer gas molecules).
- Moderate Temperature (≈ 450 °C): compromise between rate and yield.
- Iron Catalyst: speeds up both reactions → equilibrium reached faster.
Example
For \( \mathrm{N_2 + 3H_2 \rightleftharpoons 2NH_3 + Energy} \), predict the effect of adding more hydrogen gas.
▶️ Answer / Explanation
Step 1: Adding more \( \mathrm{H_2} \) increases reactant concentration.
Step 2: System shifts right to use up added \( \mathrm{H_2} \).
Final Answer: Equilibrium shifts to the right → more ammonia forms.
Example
For \( \mathrm{2SO_2 + O_2 \rightleftharpoons 2SO_3 + Energy} \), what happens when temperature increases?
▶️ Answer / Explanation
Step 1: Forward reaction is exothermic (releases heat).
Step 2: Increasing temperature adds heat → system shifts to absorb it.
Step 3: Endothermic (reverse) reaction favoured.
Final Answer: Equilibrium shifts left → less \( \mathrm{SO_3} \) forms.
Example
The equilibrium \( \mathrm{2NOCl(g) \rightleftharpoons 2NO(g) + Cl_2(g)} \) has ΔH = +75 kJ mol⁻¹. Predict the direction of equilibrium shift when (a) pressure is increased, (b) temperature is increased.
▶️ Answer / Explanation
(a) Increasing pressure favours the side with fewer gas moles.
Left side: 2 mol ; Right side: 3 mol → Shifts left (toward 2 mol).
(b) Forward reaction is endothermic (ΔH > 0). Increasing temperature favours endothermic direction → Shifts right.
Final Answer: (a) Shifts left (higher pressure). (b) Shifts right (higher temperature).
