IB MYP 4-5 Chemistry -Solutions, colloids, and suspensions- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Solutions, colloids, and suspensions- Study Notes
Key Concepts
- Solutions, Colloids, and Suspensions
Solutions, Colloids, and Suspensions
Solutions, Colloids, and Suspensions
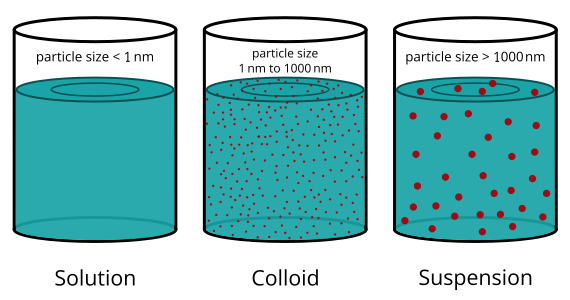
Mixtures can be classified into three main categories based on the size of their particles and how these particles behave in a solvent. These are: solutions, colloids, and suspensions.
Solution
A solution is a homogeneous mixture of two or more substances in which the particles are evenly distributed at the molecular or ionic level.
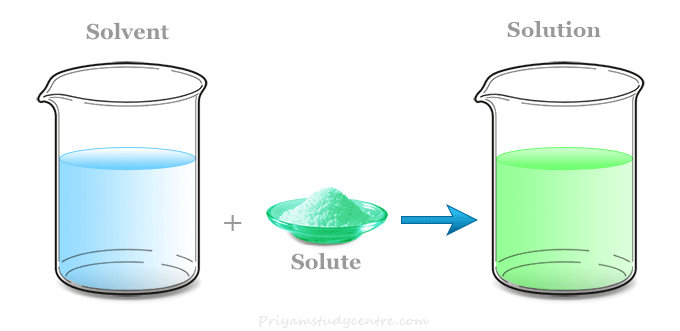
- It consists of a solute (the substance dissolved) and a solvent (the substance doing the dissolving).
- Particles are extremely small (less than 1 nm in diameter).
- The solute cannot be separated by filtration or settling.
- Solutions are transparent and allow light to pass through without scattering.
Examples:
- Salt water (salt + water)
- Sugar solution
- Air (gas solution — oxygen dissolved in nitrogen)
Key Characteristics of Solutions
- Homogeneous (uniform throughout).
- Stable — solute does not settle on standing.
- Cannot be separated by filtration.
- Do not scatter light (no Tyndall effect).
Colloid
A colloid is a heterogeneous mixture in which very small particles of one substance are evenly dispersed throughout another. The particle size is intermediate between those in a solution and a suspension (1 nm – 1000 nm).
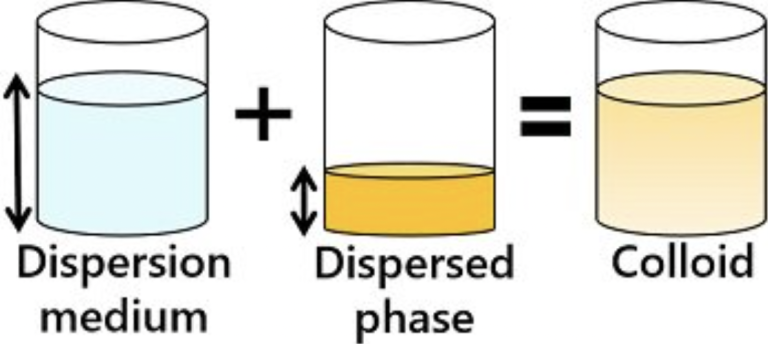
- Colloidal particles are too small to settle but large enough to scatter light.
- This scattering of light by colloidal particles is called the Tyndall effect.
- Colloids appear uniform to the naked eye but are actually heterogeneous under magnification.
Examples:
- Milk (fat droplets in water)
- Fog (water droplets in air)
- Jelly, butter, paint, smoke
Key Characteristics of Colloids
- Appear homogeneous but are microscopically heterogeneous.
- Particles do not settle on standing.
- Cannot be separated by ordinary filtration but can be separated by centrifugation.
- Exhibit Tyndall effect — scattering of light.
Suspension
A suspension is a heterogeneous mixture in which the particles of the dispersed substance are large enough to be seen and will settle out on standing.
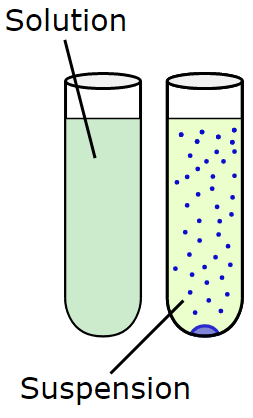
- Particle size is larger than 1000 nm.
- Particles are visible to the naked eye or under a simple microscope.
- Suspensions are opaque and scatter light strongly.
Examples:
- Sand in water
- Chalk in water
- Flour in water
- Muddy water
Key Characteristics of Suspensions
- Heterogeneous (non-uniform composition).
- Particles settle on standing (unstable).
- Can be separated by filtration.
- Scatter light (Tyndall effect present when freshly mixed).
Comparison Table: Solutions, Colloids, and Suspensions
| Property | Solution | Colloid | Suspension |
|---|---|---|---|
| Type of Mixture | Homogeneous | Heterogeneous (appears uniform) | Heterogeneous |
| Particle Size | Less than 1 nm | 1–1000 nm | Greater than 1000 nm |
| Settling on Standing | Do not settle | Do not settle | Settle on standing |
| Filtration | Cannot be filtered | Cannot be filtered by ordinary methods | Can be separated by filtration |
| Tyndall Effect | Absent | Present | Present (strong) |
| Stability | Stable | Relatively stable | Unstable |
| Examples | Salt water, sugar solution | Milk, fog, paint | Chalk in water, muddy water |
The Tyndall Effect
The Tyndall effect is the scattering of light by particles in a colloid or a suspension. It helps distinguish between true solutions and colloids.
- Seen in: Colloids and suspensions (particles large enough to scatter light).
- Not seen in: True solutions (particles too small).
Example: A beam of light passing through fog or milk becomes visible due to the Tyndall effect, but it remains invisible in a salt solution.
Example :
How would you distinguish between a solution, a colloid, and a suspension using light?
▶️ Answer / Explanation
Step 1: Pass a narrow beam of light through each mixture in a dark room.
Step 2: Observe whether the light beam is visible or not.
- Solution: Beam not visible → no Tyndall effect.
- Colloid: Beam visible → light scattered by particles.
- Suspension: Beam visible and cloudy → large particles scatter light strongly.
Final Answer: The visibility of the light beam (Tyndall effect) distinguishes the three types of mixtures.
Example :
Why is milk classified as a colloid and not a solution or a suspension?
▶️ Answer / Explanation
Step 1: Milk contains tiny fat droplets dispersed evenly in water.
Step 2: The droplets are too small to settle and too large to form a true solution.
Step 3: Milk scatters light (shows Tyndall effect) but appears uniform to the eye.
Final Answer: Milk is a colloid because its dispersed particles remain suspended and scatter light, though the mixture appears uniform.
Example :
Explain why muddy water is classified as a suspension, and describe what happens when it is left undisturbed for some time.
▶️ Answer / Explanation
Step 1: Muddy water has large soil or clay particles mixed with water.
Step 2: The particles are visible, do not dissolve, and make the mixture cloudy.
Step 3: On standing, gravity causes the heavy particles to settle at the bottom.
Final Answer: Muddy water is a suspension because its particles are large enough to settle out on standing and can be separated by filtration.
