IB MYP 4-5 Chemistry - States of matter: solids, liquids, gases- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -States of matter: solids, liquids, gases- Study Notes
Key Concepts
- States of Matter: Solids
- States of Matter: Liquids
- States of Matter: Gases
States of Matter: Solids
States of Matter: Solids, Liquids, and Gases
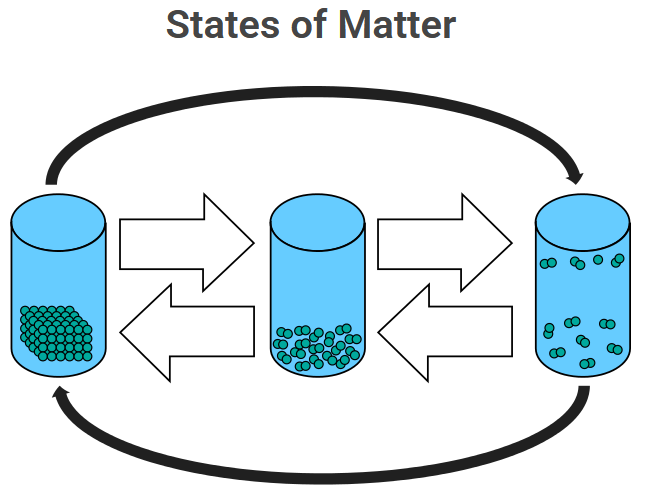
All matter is made up of tiny particles (atoms, ions, or molecules). The way these particles are arranged and how they move determines the state of matter. The three common states solid, liquid, and gas differ in particle arrangement, movement, and energy.
Definition: A state of matter is a physical form that matter can exist in, determined by the arrangement and movement of its particles and the energy they possess.
States of Matter: Solids
A solid is a state of matter in which particles are closely packed together in fixed positions and can only vibrate in place. The strong attractive forces between particles give solids a definite shape and volume.
Particle Arrangement and Movement:![]()
- Particles are arranged in a regular, repeating pattern called a lattice.
- They are very close together and held by strong forces of attraction.
- Particles can only vibrate around their fixed positions; they cannot move freely.
Energy and Forces:
- Solids have the lowest kinetic energy of all states of matter.
- The potential energy is low because the particles are already close together.
- Interparticle (intermolecular or ionic) forces are very strong, keeping particles locked in place.
Properties of Solids:
- Fixed shape and fixed volume.
- Incompressible (cannot be squashed easily).
- High density due to tightly packed particles.
- Do not flow; they retain their shape unless a force breaks them apart.
Example :
Explain why ionic solids like sodium chloride (NaCl) are hard and have high melting points, while molecular solids like iodine (I₂) are softer and melt more easily.
▶️ Answer/Explanation
Step 1: Ionic solids (e.g., NaCl) have particles held together by strong electrostatic forces between oppositely charged ions.
Step 2: These forces require large amounts of energy to overcome, giving ionic solids high melting points and hardness.
Step 3: Molecular solids (e.g., I₂) are held together by weak intermolecular (van der Waals) forces, which are much easier to overcome.
Final Answer: NaCl is hard and has a high melting point because of strong ionic bonding, while I₂ is soft and melts easily due to weak intermolecular forces.
States of Matter: Liquids
States of Matter: Liquids
A liquid is a state of matter where particles are close together but can move past each other freely. Liquids have a definite volume but no fixed shape — they take the shape of their container.
Particle Arrangement and Movement:![]()
- Particles are close together with small spaces between them.
- They are not in fixed positions and can slide or flow past each other.
- Intermolecular forces are weaker than in solids but still strong enough to keep particles close.
Energy and Forces:
- Liquids have more kinetic energy than solids but less than gases.
- As temperature increases, particle movement becomes more rapid, reducing intermolecular attraction.
- Liquids can evaporate when some surface particles gain enough energy to escape into the gas phase.
Properties of Liquids:
- No fixed shape but definite volume.
- Almost incompressible (particles are already close).
- Flow easily due to mobile particles.
- Exhibit surface tension and viscosity due to intermolecular attractions.
Example :
Explain why water droplets form spherical shapes on a leaf surface.
▶️ Answer/Explanation
Step 1: In liquids, cohesive forces (between like molecules) create surface tension.
Step 2: Water molecules at the surface experience stronger inward attraction than outward forces.
Step 3: The surface area is minimized by forming a spherical shape, which has the smallest area for a given volume.
Final Answer: Water droplets form spheres due to surface tension caused by cohesive intermolecular forces among water molecules.
States of Matter: Gases
States of Matter: Gases
A gas is a state of matter in which particles are far apart and move rapidly and randomly. Gases have neither a definite shape nor a definite volume; they expand to fill any container.
Particle Arrangement and Movement:![]()
- Particles are widely spaced and move independently.
- Intermolecular forces are negligible (almost zero).
- Particles move in straight lines until they collide elastically with other particles or the walls of the container.
Energy and Forces:
- Gases have the highest kinetic energy among all states of matter.
- The kinetic energy is directly proportional to the absolute temperature: \( \mathrm{KE_{avg} = \dfrac{3}{2}RT} \)
- Weak forces allow gases to compress easily and expand rapidly when heated.
Properties of Gases:
- No fixed shape and no fixed volume.
- Highly compressible and low density.
- Diffuse and mix rapidly with other gases.
- Exert pressure on container walls due to continuous collisions.
Example :
A sealed container of air is heated from 300 K to 600 K at constant volume. What happens to the pressure inside, assuming ideal gas behavior?
▶️ Answer/Explanation
Step 1: For a fixed volume and number of gas particles, pressure is proportional to temperature: \( \mathrm{\dfrac{P_2}{P_1} = \dfrac{T_2}{T_1}} \).
Step 2: \( \mathrm{\dfrac{P_2}{P_1} = \dfrac{600}{300} = 2} \).
Step 3: The pressure doubles when temperature doubles (in kelvin).
Final Answer: The gas pressure becomes twice its original value because particle collisions become more frequent and forceful as kinetic energy increases.
Comparison of the Three States of Matter
![]()
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Particle Arrangement | Closely packed in a fixed lattice | Close but random | Far apart and random |
| Forces Between Particles | Very strong | Moderate | Negligible |
| Kinetic Energy | Lowest | Intermediate | Highest |
| Shape and Volume | Fixed shape and volume | Takes container shape, fixed volume | No fixed shape or volume |
| Compressibility | Very low | Low | High |
| Density | High | Moderate | Low |
Example :
When the same amount of heat energy is supplied to 100 g of ice, 100 g of liquid water, and 100 g of water vapor, the rise in temperature is not the same for all three. Explain why this happens and what it reveals about the physical properties of solids, liquids, and gases.
▶️ Answer / Explanation
Observation
- When equal heat energy is given, the temperature of water vapor rises the most, liquid water rises moderately, and ice rises the least.
Concept of Heat Capacity
- The amount of temperature change depends on a material’s specific heat capacity — the energy required to raise the temperature of 1 g by 1°C.
- Substances with higher heat capacities experience smaller temperature increases for the same heat input.
Relationship Across States
- Solids (ice) have strong bonding and fixed structure, so more energy goes into increasing potential energy (loosening structure) rather than raising temperature.
- Liquids (water) require moderate energy to increase temperature because the molecules are freer but still somewhat cohesive.
- Gases (steam) have minimal internal bonding, so nearly all added energy directly increases the motion of molecules, leading to a larger temperature rise.
What This Reveals
- Different states of matter respond differently to heat due to differences in how energy is distributed within them.
- Solids absorb heat mostly to weaken internal structure, liquids partly for structure and partly for motion, and gases almost entirely to increase temperature (kinetic energy).
Conclusion
This comparison shows that the same amount of energy causes different temperature changes in solids, liquids, and gases because their internal energy storage and molecular freedom differ. It demonstrates that the thermal behavior of matter depends on its physical state, not just on the amount of energy absorbed.
Final Answer: The unequal rise in temperature reveals that gases warm up fastest, liquids moderately, and solids slowest when given equal heat — showing fundamental differences in their ability to absorb and distribute energy across the three states of matter.
