IB MYP 4-5 Chemistry -Structural Formula, Condensed Formula, and Isomerism- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Structural Formula, Condensed Formula, and Isomerism- Study Notes
Key Concepts
- Structural Formula, Condensed Formula, and Isomerism
Structural Formula, Condensed Formula, and Isomerism
Structural Formula, Condensed Formula, and Isomerism
Organic compounds can be represented in several ways to show how atoms are arranged and connected. The structure of a molecule influences its physical and chemical properties, even when the molecular formula is the same.
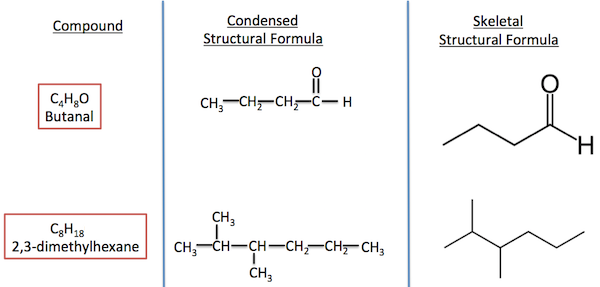
Types of Chemical Formulas in Organic Chemistry
| Type of Formula | Description | Example (Ethane) |
|---|---|---|
| Molecular Formula | Shows the total number of each type of atom in a molecule. | \( \mathrm{C_2H_6} \) |
| Condensed Structural Formula | Shows the atoms in sequence but without drawing all bonds. | \( \mathrm{CH_3CH_3} \) |
| Displayed (Structural) Formula | Shows every atom and bond clearly, illustrating connectivity. | H–C–C–H with all H atoms shown around each C |
| Skeletal Formula | Shows carbon-carbon bonds as lines, omitting hydrogen atoms attached to carbon. | Simplified zigzag line (each vertex = a carbon atom) |
Isomerism — The Existence of Compounds with the Same Formula but Different Structures
Isomers are compounds that have the same molecular formula but different structural arrangements of atoms.
These differences cause different physical and chemical properties even though their composition is the same.
Types of Isomerism (at MYP Level)
- Structural Isomerism: Atoms are connected in different ways (different structures).
- Positional Isomerism: The functional group or double bond is in a different position on the same carbon chain.
- Functional Group Isomerism: Compounds have the same molecular formula but different functional groups.
Structural Isomerism
Definition: Occurs when the same molecular formula forms compounds with different arrangements of carbon atoms (different structures).
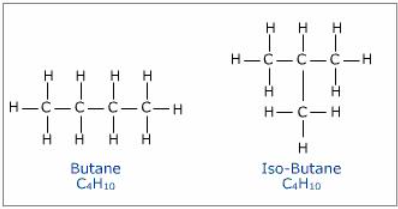
Example: For \( \mathrm{C_4H_{10}} \):
- n-butane: \( \mathrm{CH_3CH_2CH_2CH_3} \) — straight chain
- isobutane: \( \mathrm{CH_3CH(CH_3)CH_3} \) — branched chain
Key Idea: Changing the carbon skeleton gives rise to structural isomers.
Positional Isomerism
Definition: Occurs when the same functional group is attached to different carbon atoms in the chain.

Example:
- Butan-1-ol: \( \mathrm{CH_3CH_2CH_2CH_2OH} \)
- Butan-2-ol: \( \mathrm{CH_3CH_2CH(OH)CH_3} \)
Key Idea: Position of –OH group changes, but molecular formula remains \( \mathrm{C_4H_{10}O} \).
Functional Group Isomerism
Definition: Occurs when compounds have the same molecular formula but contain different functional groups.
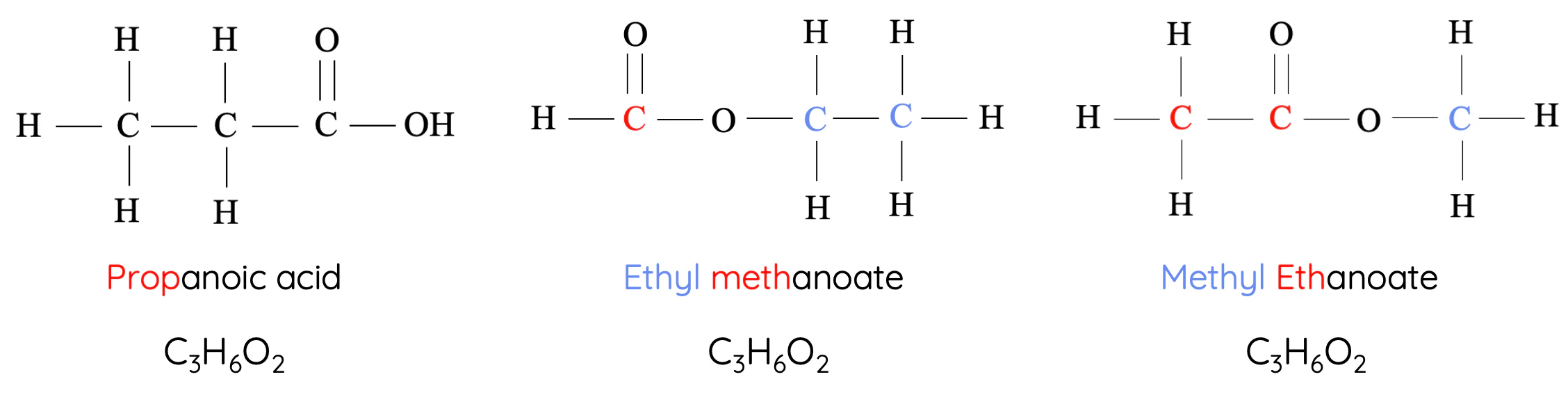
Example:
- Ethanol: \( \mathrm{CH_3CH_2OH} \) → Alcohol group (–OH)
- Dimethyl ether: \( \mathrm{CH_3OCH_3} \) → Ether group (–O–)
Key Idea: Same molecular formula (\( \mathrm{C_2H_6O} \)), different functional groups = different chemical properties.
Importance of Isomerism
- Explains the diversity of organic compounds.
- Isomers can have different boiling points, solubility, or reactivity.
- Essential in pharmaceuticals — different isomers may have different biological effects.
Summary
- Organic compounds can be represented using molecular, condensed, structural, or skeletal formulas.
- Isomerism occurs when compounds have the same molecular formula but different structures.
- Types of isomerism:
- Structural: Different carbon skeletons
- Positional: Same group, different position
- Functional: Different functional groups
- Isomerism leads to great diversity in organic compounds.
Isomerism shows that the same atoms can form different molecules with unique properties structure defines behavior in organic chemistry.
Example
Write all structural isomers of butane (\( \mathrm{C_4H_{10}} \)).
▶️ Answer / Explanation
Step 1: Total carbon = 4, total hydrogen = 10.
Step 2: Possible structures:
- \( \mathrm{CH_3CH_2CH_2CH_3} \) — n-butane
- \( \mathrm{CH_3CH(CH_3)CH_3} \) — isobutane (2-methylpropane)
Final Answer: Two structural isomers: n-butane and 2-methylpropane.
Example
Identify the type of isomerism between butan-1-ol (\( \mathrm{CH_3CH_2CH_2CH_2OH} \)) and butan-2-ol (\( \mathrm{CH_3CH_2CH(OH)CH_3} \)).
▶️ Answer / Explanation
Step 1: Both compounds have same molecular formula \( \mathrm{C_4H_{10}O} \).
Step 2: The position of the –OH group changes (on carbon 1 vs carbon 2).
Step 3: Functional group remains the same.
Final Answer: Positional isomerism.
Example
Explain how ethanol (\( \mathrm{CH_3CH_2OH} \)) and dimethyl ether (\( \mathrm{CH_3OCH_3} \)) can be isomers of each other.
▶️ Answer / Explanation
Step 1: Both have molecular formula \( \mathrm{C_2H_6O} \).
Step 2: Ethanol has an alcohol group (–OH), while dimethyl ether has an ether linkage (–O–).
Step 3: Their structures and properties differ.
Final Answer: They are functional group isomers — same molecular formula, different functional groups.
