IB MYP 4-5 Chemistry -Subatomic particles — protons, neutrons, electrons- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Subatomic particles — protons, neutrons, electrons- Study Notes
Key Concepts
- Subatomic Particles — Protons, Neutrons, and Electrons
Subatomic Particles — Protons, Neutrons, and Electrons
Subatomic Particles — Protons, Neutrons, and Electrons
Every atom is made up of even smaller particles called subatomic particles. These are the fundamental particles that determine the atom’s mass, charge, and chemical behavior. The three main subatomic particles are protons, neutrons, and electrons.

Overview of Subatomic Particles
Each subatomic particle has unique properties such as charge, mass, and location within the atom.
| Particle | Symbol | Relative Charge | Relative Mass | Location in Atom | Role / Function |
|---|---|---|---|---|---|
| Proton | \( \mathrm{p^+} \) | +1 | 1 | Nucleus | Determines element identity (atomic number) |
| Neutron | \( \mathrm{n^0} \) | 0 | 1 | Nucleus | Adds mass and stabilizes the nucleus |
| Electron | \( \mathrm{e^-} \) | −1 | 1/1836 (very small) | Electron shells (outside nucleus) | Involved in bonding and reactions |
Proton \(( \mathrm{p^+} )\)
A proton is a positively charged subatomic particle found in the nucleus of an atom.
- Charge: +1 (same magnitude as electron’s negative charge)
- Mass: 1 atomic mass unit (a.m.u.)
- Determines the identity of the element (atomic number = number of protons).
Example:
- Hydrogen atom → 1 proton → atomic number = 1
- Oxygen atom → 8 protons → atomic number = 8
Changing the number of protons changes the element itself. (e.g., 6 protons = carbon, 7 protons = nitrogen)
Neutron \(( \mathrm{n^0} )\)
A neutron is a neutral particle (no charge) found in the nucleus, almost the same mass as a proton.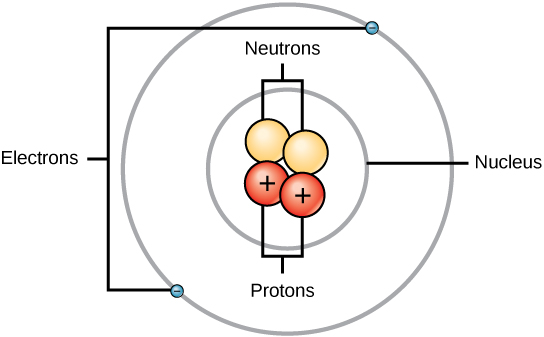
- Charge: 0 (neutral)
- Mass: 1 a.m.u.
- Provides stability to the nucleus by balancing proton repulsion.
Example:
- Carbon-12 → 6 protons, 6 neutrons
- Carbon-14 → 6 protons, 8 neutrons (radioactive isotope)
Atoms of the same element with different numbers of neutrons are called isotopes.
Electron \(( \mathrm{e^-} )\)
An electron is a negatively charged particle that moves around the nucleus in energy levels (shells).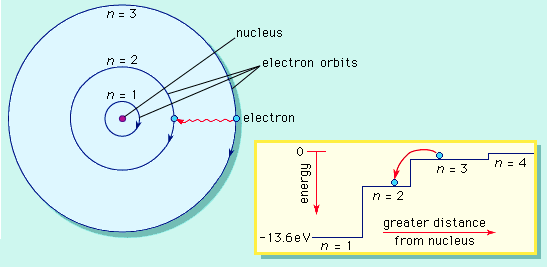
- Charge: −1
- Mass: Negligible (1/1836 a.m.u.)
- Responsible for chemical bonding and reactivity.
Energy Levels:
- Electrons occupy shells (K, L, M, N…) around the nucleus.
- Maximum electrons in a shell = \( \mathrm{2n^2} \), where \( \mathrm{n} \) = shell number.
- Example: K shell (n=1) → 2 electrons; L shell (n=2) → 8 electrons.
Chemical properties of an atom depend on the arrangement of electrons, especially the outermost ones (valence electrons).
Relationship Between Subatomic Particles
The number and arrangement of subatomic particles define the type, mass, and charge of an atom.
| Quantity | Formula / Relation | Meaning |
|---|---|---|
| Atomic Number (\( \mathrm{Z} \)) | Number of protons = Number of electrons | Defines the element and its position in the periodic table |
| Mass Number (\( \mathrm{A} \)) | \( \mathrm{A = p + n} \) | Sum of protons and neutrons in the nucleus |
| Neutrons (\( \mathrm{n} \)) | \( \mathrm{n = A – Z} \) | Difference between mass number and atomic number |
Atomic Model Summary
- Protons and neutrons form the dense, positively charged nucleus.
- Electrons move in energy levels (shells) around the nucleus.
- Atoms are mostly empty space.
- Overall atom is neutral because total positive = total negative charge.
Example :
An atom of oxygen has atomic number 8 and mass number 16. Determine the number of protons, neutrons, and electrons.
▶️ Answer / Explanation
Step 1: \( \mathrm{Z = 8 \Rightarrow 8\ protons,\ 8\ electrons} \)
Step 2: \( \mathrm{A = 16 \Rightarrow neutrons = 16 – 8 = 8} \)
Final Answer: Protons = 8, Neutrons = 8, Electrons = 8
Example:
What happens to the number of subatomic particles when a neutral sodium atom forms a Na⁺ ion?
▶️ Answer / Explanation
Step 1: A neutral sodium atom has 11 protons and 11 electrons.
Step 2: When it loses one electron → Na⁺ ion.
Step 3: Protons = 11 (unchanged), Electrons = 10, Neutrons = unchanged.
Final Answer: The Na⁺ ion has one fewer electron than the atom, giving it a positive charge.
Example :
Carbon-12 and Carbon-14 are isotopes of carbon. What do they have in common, and how do they differ?
▶️ Answer / Explanation
Step 1: Both have the same number of protons (6) → same element.
Step 2: Carbon-12 has 6 neutrons, while Carbon-14 has 8 neutrons.
Step 3: They have identical chemical properties (same electrons) but different masses and stability.
Final Answer: Isotopes have equal protons and electrons but differ in neutron number, giving different mass numbers.
