IB MYP 4-5 Chemistry -Sustainability and recycling of materials- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Sustainability and recycling of materials- Study Notes
Key Concepts
- Sustainability and Recycling of Materials
- Green Chemistry and Sustainable Development
Sustainability and Recycling of Materials
Sustainability and Recycling of Materials
Sustainability means meeting the needs of the present generation without compromising the ability of future generations to meet their own needs. In chemistry, it involves using natural resources responsibly, minimizing waste, and protecting the environment.
Recycling is the process of collecting, processing, and reusing materials that would otherwise be thrown away as waste — turning them into new products.
Importance of Sustainability in Chemistry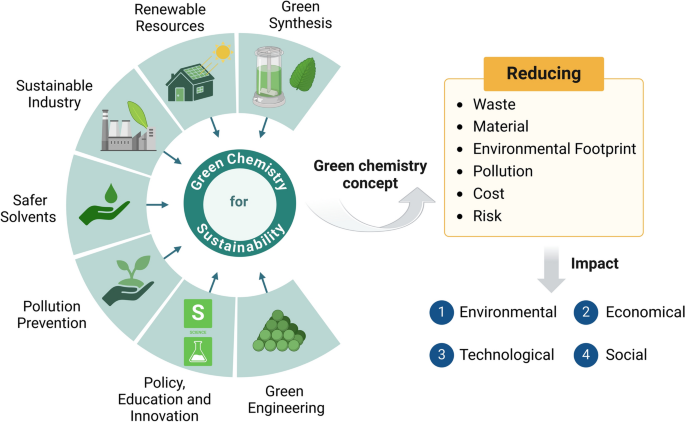
Chemistry plays a crucial role in creating a sustainable future by:
- Developing renewable materials and energy sources.
- Designing green chemical processes that reduce waste and pollution.
- Promoting efficient use of natural resources such as metals, water, and fossil fuels.
Key Idea: Sustainability focuses on balancing economic growth, environmental protection, and social well-being.
The Three Rs of Sustainability

| Principle | Meaning | Examples |
|---|---|---|
| Reduce | Use fewer resources and generate less waste. | Using reusable bottles instead of disposable plastic ones. |
| Reuse | Use items multiple times before discarding them. | Reusing glass jars for storage. |
| Recycle | Convert waste into new usable materials. | Recycling aluminum cans and paper. |
Recycling of Common Materials
(a) Metals:
- Metals like aluminum, copper, and iron are melted and purified for reuse.
- Recycling metals saves energy — for example, recycling aluminum saves up to 95% of the energy required to produce it from ore.
Equation (Example: Extraction vs Recycling)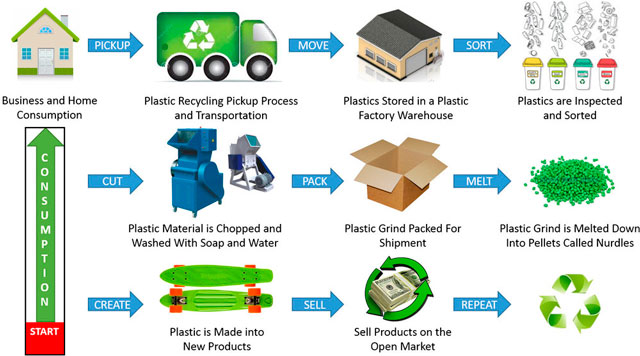
\( \mathrm{2Al_2O_3 \xrightarrow{electrolysis} 4Al + 3O_2} \)
Recycling skips this energy-intensive process, making it more sustainable.
(b) Glass:
- Glass is crushed, melted, and reformed into new bottles or containers.
- Recycling glass reduces landfill waste and conserves raw materials like sand and limestone.
(c) Paper:
- Old paper is pulped, cleaned, and pressed into new sheets.
- Recycling paper reduces deforestation and water consumption.
(d) Plastics:
- Sorted by type, shredded, and melted to make new products.
- Plastics like PET (polyethylene terephthalate) can be recycled into fibers or containers.
Equation (Example: Polymerization of Ethene):
\( \mathrm{nCH_2=CH_2 \rightarrow (CH_2-CH_2)_n} \)
Recycling reduces the accumulation of non-biodegradable polymers.
Benefits of Recycling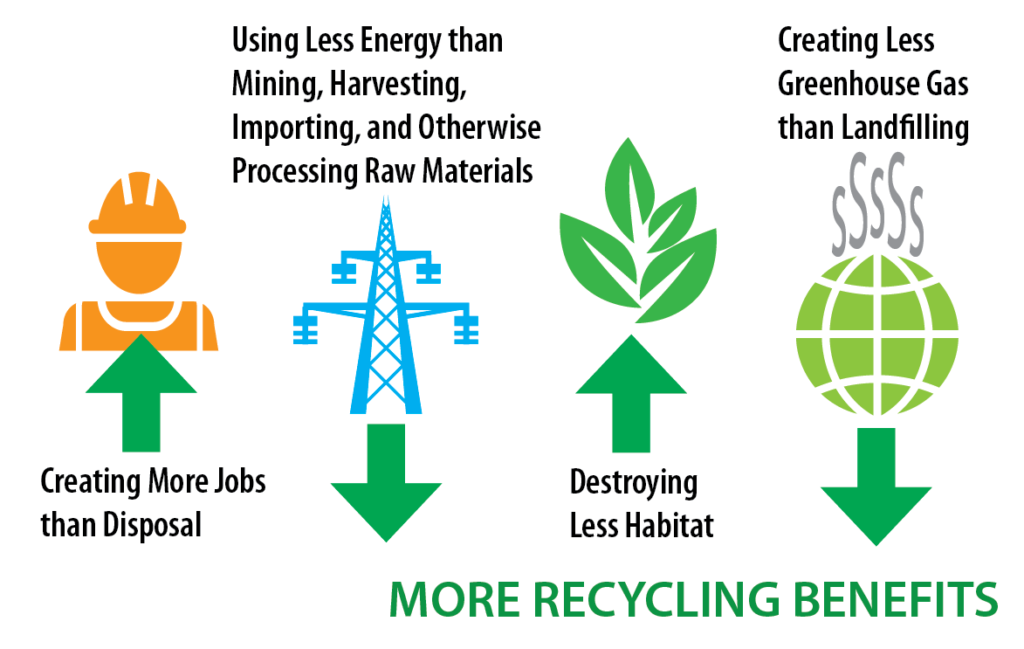
- Conserves natural resources and raw materials.
- Reduces energy consumption and greenhouse gas emissions.
- Decreases landfill waste and pollution.
- Creates employment in recycling and waste management sectors.
Challenges in Recycling
- Sorting materials efficiently can be difficult and costly.
- Contaminated waste reduces recycling efficiency.
- Some plastics and composite materials cannot be easily recycled.
- Energy is still required in the recycling process (though less than raw extraction).
Sustainable Use of Resources
- Using renewable resources (solar, wind, biomass) instead of non-renewable (coal, oil, gas).
- Designing products with biodegradable materials and recyclable packaging.
- Encouraging green chemistry — minimizing the use and production of hazardous substances.
- Circular economy: Materials are reused continuously rather than disposed of, creating a sustainable loop.
Recycling Overview
| Material | Recycling Method | Benefit |
|---|---|---|
| Aluminum | Melt and reform | Saves 95% of energy compared to mining |
| Glass | Crushed and remelted | Conserves raw materials |
| Paper | Pulping and pressing | Reduces deforestation |
| Plastics (PET) | Melted and reformed | Reduces non-biodegradable waste |
Example
Why is recycling aluminum considered more sustainable than extracting it from bauxite?
▶️ Answer / Explanation
Step 1: Extraction from bauxite by electrolysis consumes large amounts of electricity.
Step 2: Recycling aluminum uses about 95% less energy.
Step 3: It reduces environmental pollution and conserves resources.
Final Answer: Recycling is more sustainable as it saves energy and reduces mining waste.
Example
Explain why not all plastics can be recycled easily.
▶️ Answer / Explanation
Step 1: Plastics have different chemical structures and melting points.
Step 2: Thermoplastics (like PET) can be melted and reformed, but thermosetting plastics cannot be remelted once hardened.
Step 3: Mixed or contaminated plastics are difficult to separate efficiently.
Final Answer: Some plastics are chemically cross-linked or impure, making recycling inefficient or impossible.
Example
Discuss how the principles of green chemistry can improve sustainability in chemical industries.
▶️ Answer / Explanation
Step 1: Green chemistry focuses on reducing waste, using renewable feedstocks, and avoiding toxic substances.
Step 2: Example: Using catalysts to lower energy use or replacing harmful solvents with eco-friendly ones.
Step 3: Sustainable production reduces environmental damage and conserves resources.
Final Answer: Green chemistry enhances sustainability by designing safer, energy-efficient, and less polluting chemical processes.
Green Chemistry and Sustainable Development
Green Chemistry and Sustainable Development
Green chemistry is the branch of chemistry that focuses on designing chemical products and processes that minimize or eliminate the use and generation of hazardous substances. It aims to make chemical manufacturing safer, cleaner, and more efficient — helping achieve sustainable development.
Sustainable development is meeting the needs of the present without compromising the ability of future generations to meet their own needs. Green chemistry supports sustainability by reducing pollution, conserving energy, and using renewable resources.
Goals of Green Chemistry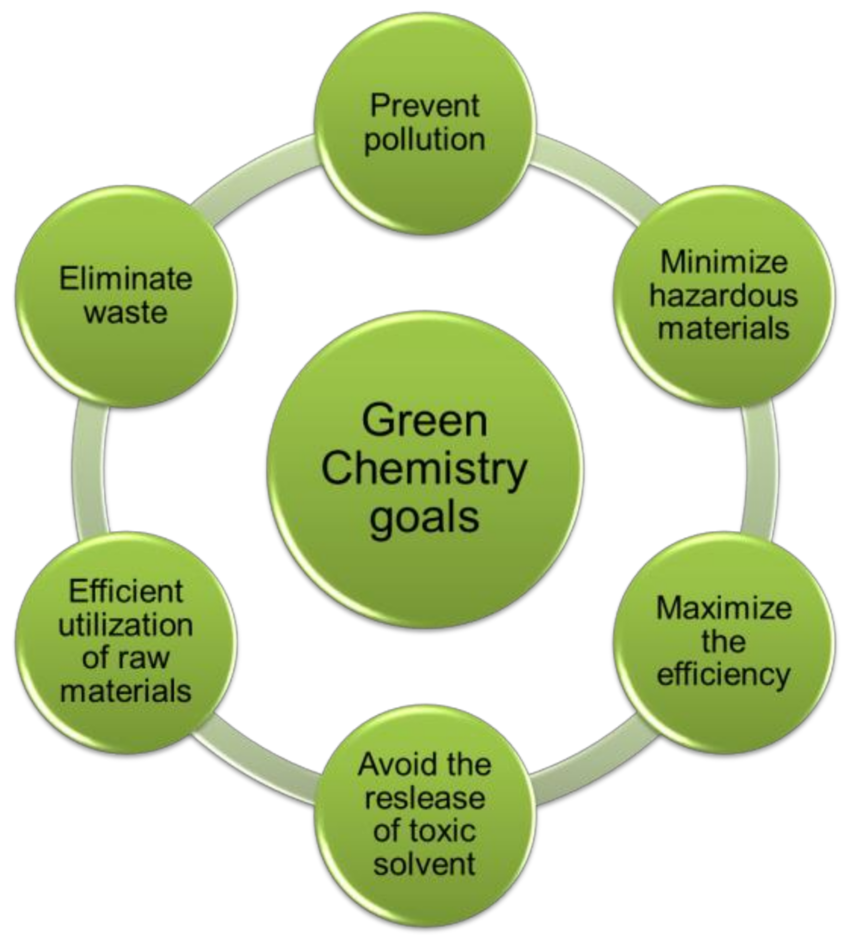
- Prevent pollution and reduce hazardous waste at the source.
- Use renewable raw materials instead of non-renewable ones.
- Develop energy-efficient and safe chemical processes.
- Design biodegradable and non-toxic products.
- Promote economic and environmental balance.
Key Idea: Green chemistry integrates environmental protection directly into the design of chemical products and processes — not just cleaning up pollution after it occurs.
The Twelve Principles of Green Chemistry
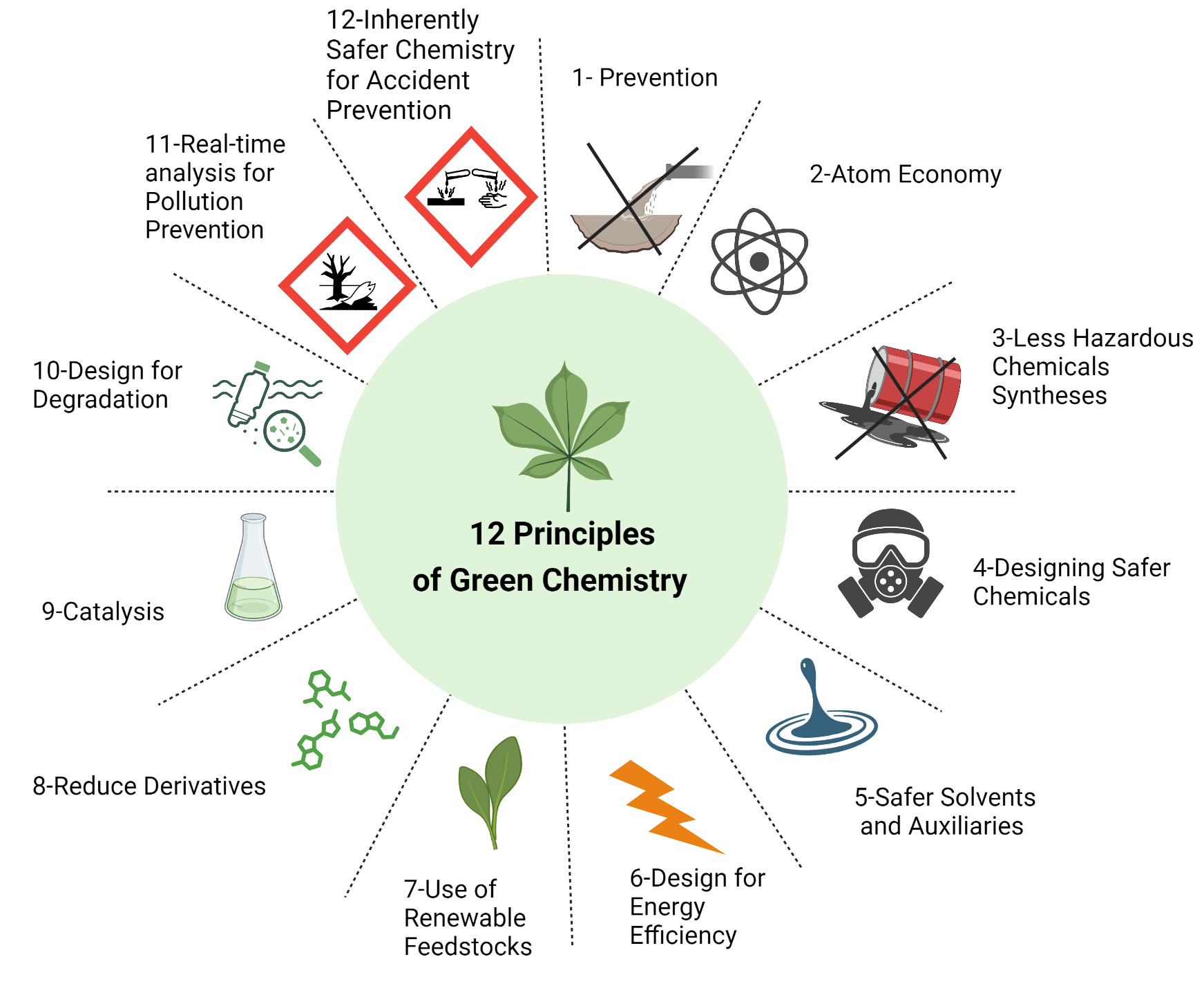
| Principle | Explanation |
|---|---|
| 1. Prevention | It is better to prevent waste than to treat or clean it up later. |
| 2. Atom Economy | Design reactions so that all atoms in the starting materials end up in the final product. |
| 3. Less Hazardous Chemicals | Use or produce substances that are not toxic to humans or the environment. |
| 4. Safer Solvents | Avoid harmful solvents; use water or ethanol instead of toxic ones. |
| 5. Energy Efficiency | Carry out reactions at room temperature and pressure to save energy. |
| 6. Renewable Feedstocks | Use renewable raw materials like plant-based ethanol instead of petroleum. |
| 7. Catalysis | Use catalysts to speed reactions and reduce waste, instead of excess reagents. |
| 8. Reduce Derivatives | Avoid unnecessary steps like using protecting groups that generate waste. |
| 9. Biodegradable Products | Design chemicals that break down safely after use. |
| 10. Real-Time Analysis | Monitor reactions to prevent formation of by-products. |
| 11. Safety | Choose substances and processes that reduce accident risks like explosions or releases. |
| 12. Minimize Pollution | Reduce emissions and chemical waste during production. |
Examples of Green Chemistry in Action
- Bio-plastics: Made from corn starch or sugarcane instead of petroleum.
- Supercritical CO₂ as a solvent: Replaces toxic organic solvents in cleaning and extraction processes.
- Hydrogen fuel cells: Produce electricity with water as the only byproduct.
- Catalytic converters: Reduce harmful car emissions (\( \mathrm{CO,\ NO_x,\ hydrocarbons} \)).
Equation (Catalytic Converter Reaction):
\( \mathrm{2CO + 2NO \rightarrow 2CO_2 + N_2} \)
Connection Between Green Chemistry and Sustainable Development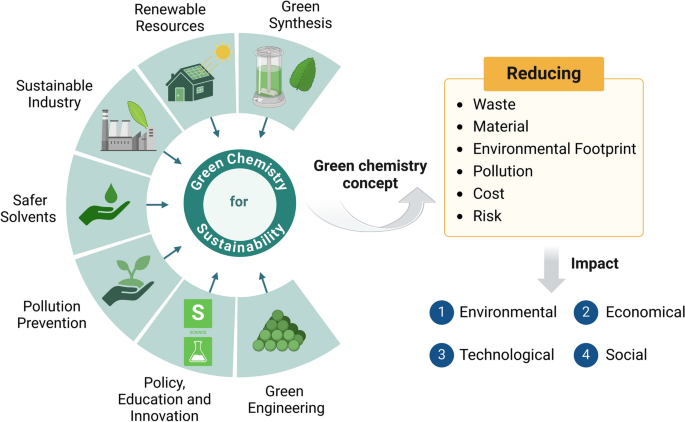
Green chemistry supports sustainable development by:
- Reducing environmental pollution and conserving ecosystems.
- Lowering energy and material consumption in industries.
- Promoting renewable energy and biodegradable materials.
- Encouraging safer manufacturing and waste reduction.
Equation Example (Bioethanol Production):
\( \mathrm{C_6H_{12}O_6 \xrightarrow{yeast} 2C_2H_5OH + 2CO_2} \)
Bioethanol is renewable, reduces carbon emissions, and supports sustainable fuel development.
Benefits of Green Chemistry
- Cleaner environment and reduced pollution.
- Safer chemical processes and workplaces.
- Lower production costs due to efficient processes.
- Conservation of non-renewable natural resources.
- Improved public health and environmental quality.
Green Chemistry Overview
| Aspect | Description |
|---|---|
| Goal | Design safer, cleaner, and more efficient chemical processes. |
| Key Focus | Pollution prevention, atom economy, renewable materials. |
| Benefits | Protects environment, reduces waste, saves energy. |
| Connection to Sustainability | Supports long-term environmental and economic balance. |
Example
Why is green chemistry considered environmentally friendly?
▶️ Answer / Explanation
Step 1: Green chemistry aims to prevent pollution rather than treat it afterward.
Step 2: It uses non-toxic materials and renewable resources.
Step 3: Processes are designed to reduce waste and energy use.
Final Answer: Green chemistry minimizes waste and hazards, making it safer for the environment and humans.
Example
Explain the importance of atom economy in green chemistry with an example.
▶️ Answer / Explanation
Step 1: Atom economy measures how efficiently atoms in reactants are used in the final product.
Step 2: Example: In the synthesis of ethanol, all atoms from glucose end up in ethanol and carbon dioxide.
Step 3: \( \mathrm{C_6H_{12}O_6 \rightarrow 2C_2H_5OH + 2CO_2} \)
Final Answer: High atom economy means less waste and more sustainable chemical processes.
Example
Discuss how green chemistry can contribute to reducing global environmental challenges like climate change and pollution.
▶️ Answer / Explanation
Step 1: Green chemistry reduces greenhouse gas emissions by using renewable energy and cleaner reactions.
Step 2: It limits toxic waste generation and promotes biodegradable materials.
Step 3: Adoption in industries leads to reduced carbon footprint and improved sustainability.
Final Answer: Green chemistry helps combat pollution and climate change by making industrial chemistry environmentally responsible and resource-efficient.
