IB MYP 4-5 Chemistry -Titrations and concentration calculations- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Titrations and concentration calculations- Study Notes
Key Concepts
- Acid–Base Titrations and Concentration Calculations
- Equivalence Point in Acid–Base Reactions
Acid–Base Titrations and Concentration Calculations
Acid–Base Titrations and Concentration Calculations
An acid–base titration is a quantitative analytical technique used to determine the unknown concentration of an acid or base by neutralizing it with a base or acid of known concentration.
Principle of Titration
Acids and bases react according to the neutralization equation: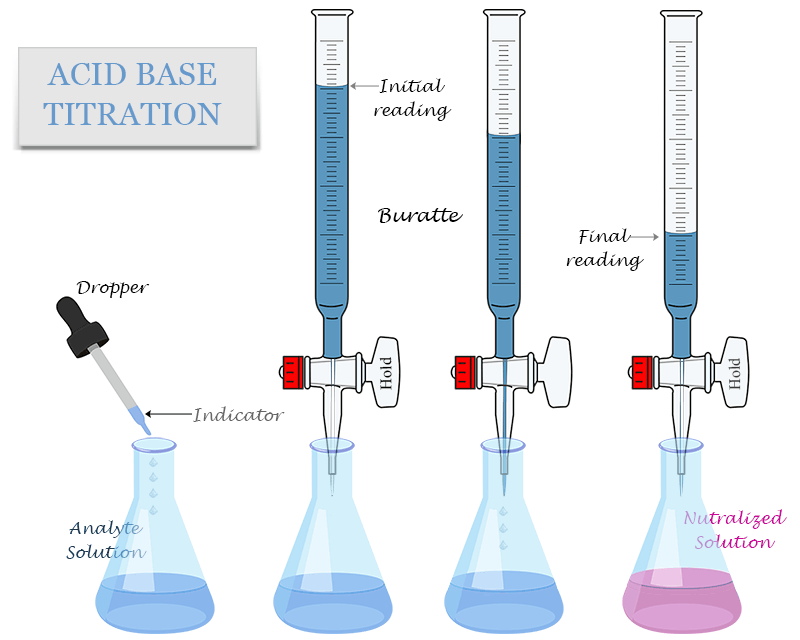
\( \mathrm{Acid + Base \rightarrow Salt + Water} \)
The reaction can be represented as:
\( \mathrm{n_1 M_1 V_1 = n_2 M_2 V_2} \)
- \( \mathrm{M_1, M_2} \): molar concentrations of acid and base
- \( \mathrm{V_1, V_2} \): volumes of acid and base used (in dm³)
- \( \mathrm{n_1, n_2} \): mole ratio from balanced equation
Common Laboratory Setup
- Burette: contains the acid or base of known concentration.
- Pipette: accurately measures a fixed volume of the unknown solution.
- Conical flask: contains the unknown solution and a few drops of an indicator.
- Indicator: shows the end point (when neutralization is complete).
Indicators Used in Acid–Base Titrations
| Type of Acid–Base Titration | Example | Indicator Used | Color Change at End Point |
|---|---|---|---|
| Strong acid + Strong base | \( \mathrm{HCl + NaOH} \) | Phenolphthalein or methyl orange | Colorless → Pink (phenolphthalein) |
| Strong acid + Weak base | \( \mathrm{HCl + NH_4OH} \) | Methyl orange | Yellow → Red |
| Weak acid + Strong base | \( \mathrm{CH_3COOH + NaOH} \) | Phenolphthalein | Colorless → Pink |
Steps in an Acid–Base Titration
- Rinse the burette with the standard solution (acid/base).
- Rinse the pipette with the unknown solution.
- Transfer the unknown solution into the conical flask and add 2–3 drops of indicator.
- Fill the burette with the standard solution.
- Slowly add solution from the burette while swirling the flask.
- Stop when the indicator changes color (end point reached).
- Record the burette reading. Repeat for concordant results.
Concentration Calculations
Formula 1: \( \mathrm{n_1 M_1 V_1 = n_2 M_2 V_2} \)
Formula 2 (Moles–Mass Relationship): \( \mathrm{n = \dfrac{m}{M_r}} \)
- \( \mathrm{n} \): number of moles
- \( \mathrm{m} \): mass (g)
- \( \mathrm{M_r} \): relative molar mass
Formula 3 (Concentration): \( \mathrm{Concentration\ (mol\,dm^{-3}) = \dfrac{moles}{volume\ (dm^3)}} \)
Example
25.0 cm³ of \( \mathrm{NaOH} \) solution exactly neutralizes 25.0 cm³ of 0.1 M \( \mathrm{HCl} \). Calculate the concentration of the sodium hydroxide solution.
▶️ Answer / Explanation
Step 1: \( \mathrm{HCl + NaOH \rightarrow NaCl + H_2O} \)
Step 2: Mole ratio = 1 : 1
Step 3: \( \mathrm{M_1V_1 = M_2V_2} \)
→ \( \mathrm{(0.1)(25) = M_2(25)} \)
→ \( \mathrm{M_2 = 0.1\ mol\,dm^{-3}} \)
Final Answer: Sodium hydroxide concentration = \( \mathrm{0.10\ mol\,dm^{-3}} \).
Example
25.0 cm³ of 0.2 M \( \mathrm{H_2SO_4} \) reacts completely with 50.0 cm³ of sodium hydroxide solution. Find the concentration of the \( \mathrm{NaOH} \) solution.
▶️ Answer / Explanation
Step 1: \( \mathrm{H_2SO_4 + 2NaOH \rightarrow Na_2SO_4 + 2H_2O} \)
Step 2: Mole ratio = 1 : 2
Step 3: \( \mathrm{M_1V_1/n_1 = M_2V_2/n_2} \)
→ \( \mathrm{(0.2)(25)/1 = M_2(50)/2} \)
→ \( \mathrm{M_2 = 0.2\ mol\,dm^{-3}} \)
Final Answer: Sodium hydroxide concentration = \( \mathrm{0.20\ mol\,dm^{-3}} \).
Example
25.0 cm³ of 0.1 M hydrochloric acid reacts with calcium hydroxide. Calculate the mass of calcium hydroxide required for neutralization.
▶️ Answer / Explanation
Step 1: \( \mathrm{2HCl + Ca(OH)_2 \rightarrow CaCl_2 + 2H_2O} \)
Step 2: Moles of \( \mathrm{HCl} = 0.1 \times \dfrac{25}{1000} = 0.0025\ mol \)
Step 3: From equation, \( \mathrm{2\ mol\ HCl} \) reacts with \( \mathrm{1\ mol\ Ca(OH)_2} \)
→ Moles of \( \mathrm{Ca(OH)_2} = \dfrac{0.0025}{2} = 0.00125\ mol \)
Step 4: \( \mathrm{Mass = n \times M_r = 0.00125 \times 74 = 0.0925\ g} \)
Final Answer: \( \mathrm{0.093\ g\ of\ Ca(OH)_2} \) required for neutralization.
Equivalence Point in Acid–Base Reactions
Equivalence Point in Acid–Base Reactions
The equivalence point in an acid–base titration is the exact point at which the amount of acid and base have reacted completely and exactly according to their stoichiometric ratio.
![]()
Difference Between Equivalence Point and End Point
Although often close, the equivalence point and end point are not the same:
| Feature | Equivalence Point | End Point |
|---|---|---|
| Definition | Exact stoichiometric neutralization of acid and base | Color change shown by indicator |
| Determination | Calculated chemically (moles of acid = moles of base × ratio) | Observed visually (indicator changes color) |
| Occurrence | Ideally exact | Close to equivalence point |
| Example | \( \mathrm{n_{acid} = n_{base}} \) | Phenolphthalein turns colorless → pink |
Determining the Equivalence Point
In a titration, the equivalence point is reached when the number of moles of hydrogen ions equals the number of moles of hydroxide ions (based on the balanced equation).
\( \mathrm{n_{acid} = n_{base} \times \dfrac{n_1}{n_2}} \)
Where:
- \( \mathrm{n_{acid}} \): moles of acid
- \( \mathrm{n_{base}} \): moles of base
- \( \mathrm{n_1, n_2} \): stoichiometric coefficients from balanced equation
pH at the Equivalence Point
The pH value at the equivalence point depends on the type of acid and base reacting:
| Type of Reaction | Example | pH at Equivalence Point | Nature of Salt Formed |
|---|---|---|---|
| Strong acid + Strong base | \( \mathrm{HCl + NaOH} \) | 7 (neutral) | Neutral salt |
| Strong acid + Weak base | \( \mathrm{HCl + NH_4OH} \) | Below 7 (acidic) | Acidic salt (e.g., \( \mathrm{NH_4Cl} \)) |
| Weak acid + Strong base | \( \mathrm{CH_3COOH + NaOH} \) | Above 7 (basic) | Basic salt (e.g., \( \mathrm{CH_3COONa} \)) |
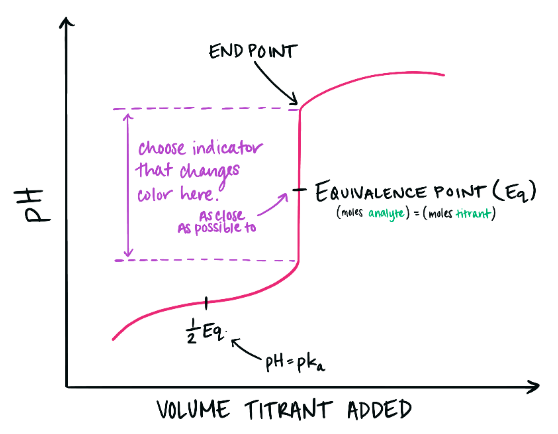
Graphical Representation — Titration Curves
A titration curve plots pH (y-axis) against volume of titrant added (x-axis). The steepest part of the curve corresponds to the equivalence point.
![]()
Characteristics:
- Sharp vertical rise for strong acid–strong base titration.
- Gradual slope for weak acid–strong base or weak base–strong acid titration.
- Indicator chosen should change color within the vertical region near the equivalence point.
Choosing a Suitable Indicator
The correct indicator depends on the pH range of the equivalence point:
| Type of Titration | pH Range at Equivalence Point | Suitable Indicator | Color Change |
|---|---|---|---|
| Strong acid + Strong base | ≈ 7 | Phenolphthalein or methyl orange | Colorless ↔ Pink or Red ↔ Yellow |
| Strong acid + Weak base | Below 7 | Methyl orange | Red ↔ Yellow |
| Weak acid + Strong base | Above 7 | Phenolphthalein | Colorless ↔ Pink |
Example
In a titration between \( \mathrm{HCl} \) and \( \mathrm{NaOH} \), what is the pH at the equivalence point and what indicator would be suitable?
▶️ Answer / Explanation
Step 1: Both acid and base are strong.
Step 2: pH at equivalence = 7 (neutral).
Step 3: Phenolphthalein or methyl orange can be used.
Final Answer: pH = 7; indicator = phenolphthalein (colorless → pink).
Example
For the titration of \( \mathrm{CH_3COOH} \) with \( \mathrm{NaOH} \), the pH at equivalence is around 8.3. Which indicator should be chosen and why?
▶️ Answer / Explanation
Step 1: Weak acid + strong base → equivalence pH > 7.
Step 2: Choose indicator that changes color in basic range (8–10).
Final Answer: Phenolphthalein (colorless → pink) is suitable since its transition range matches the equivalence pH.
Example
Explain why methyl orange is not a suitable indicator for titrating a weak acid against a strong base, using a titration curve explanation.
▶️ Answer / Explanation
Step 1: Weak acid–strong base titration → equivalence pH ≈ 8–9.
Step 2: Methyl orange changes between pH 3.1–4.4 (acidic range).
Step 3: Color change would occur long before the equivalence point.
Step 4: The titration curve rises slowly near pH 4, but steeply near pH 8–9.
Final Answer: Methyl orange gives an early end point — phenolphthalein is correct since its range (8–10) matches the equivalence point.
