IB MYP 4-5 Chemistry -Types of chemical reactions — synthesis, decomposition, displacement- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Types of chemical reactions — synthesis, decomposition, displacement- Study Notes
Key Concepts
- Types of Chemical Reactions
Types of Chemical Reactions
Types of Chemical Reactions
Chemical reactions can be classified into several types based on how reactants combine, separate, or exchange parts to form products. Each type of reaction follows a characteristic pattern and involves specific kinds of chemical changes and energy transfers.
Key Idea: Classifying reactions helps predict products, understand reactivity, and balance equations correctly.
The Five Major Types of Chemical Reactions
Synthesis (Combination) Reaction
Two or more substances combine to form a single new product.
General form: \( \mathrm{A + B \rightarrow AB} \)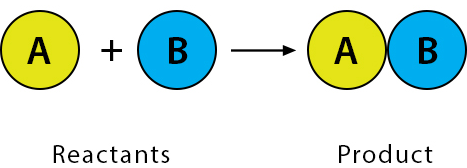
Features:
- One product formed from two or more reactants.
- Usually exothermic (energy released).
- Common in reactions of elements with oxygen or halogens.
Examples:
- \( \mathrm{2Mg + O_2 \rightarrow 2MgO} \)
- \( \mathrm{N_2 + 3H_2 \rightarrow 2NH_3} \)
- \( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \)
Decomposition Reaction
A single compound breaks down into two or more simpler products.
General form: \( \mathrm{AB \rightarrow A + B} \)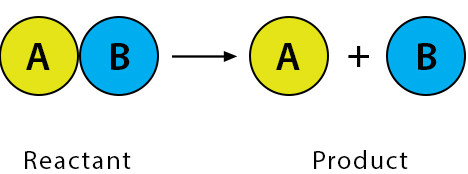
Features:
- Opposite of synthesis reaction.
- Usually endothermic (requires energy input).
- Decomposition may occur due to heat, electricity, or light.
Types of Decomposition:
| Type | Cause | Example |
|---|---|---|
| Thermal Decomposition | Heat | \( \mathrm{CaCO_3 \xrightarrow{\Delta} CaO + CO_2} \) |
| Electrolytic Decomposition | Electric current | \( \mathrm{2H_2O \xrightarrow{electricity} 2H_2 + O_2} \) |
| Photochemical Decomposition | Light | \( \mathrm{2AgCl \xrightarrow{light} 2Ag + Cl_2} \) |
Single Displacement (Replacement) Reaction
A more reactive element replaces a less reactive element in a compound.
General form: \( \mathrm{A + BC \rightarrow AC + B} \)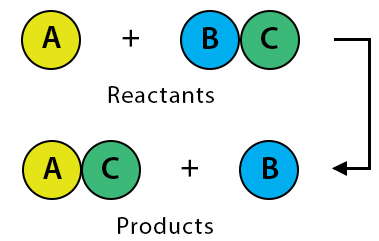
Features:
- Occurs between an element and a compound.
- Driven by differences in reactivity of elements.
- Involves metals displacing metals or halogens displacing halogens.
Examples:
- \( \mathrm{Zn + CuSO_4 \rightarrow ZnSO_4 + Cu} \)
- \( \mathrm{Cl_2 + 2NaBr \rightarrow 2NaCl + Br_2} \)
- \( \mathrm{Fe + CuSO_4 \rightarrow FeSO_4 + Cu} \)
Reactivity Series Rule: A more reactive metal replaces a less reactive one from its salt solution.
Double Displacement Reaction
A reaction where the positive and negative ions of two compounds exchange places to form two new compounds.
General form: \( \mathrm{AB + CD \rightarrow AD + CB} \)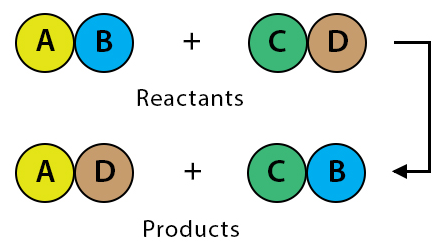
Features:
- Occurs in ionic solutions.
- Often results in precipitate formation, gas evolution, or neutralization.
- Commonly occurs between acids, bases, and salts.
Examples:
- \( \mathrm{AgNO_3 + NaCl \rightarrow AgCl↓ + NaNO_3} \)
- \( \mathrm{Na_2SO_4 + BaCl_2 \rightarrow BaSO_4↓ + 2NaCl} \)
- \( \mathrm{HCl + NaOH \rightarrow NaCl + H_2O} \)
Types of Double Displacement:
- Precipitation Reaction — formation of an insoluble solid.
- Neutralization Reaction — acid + base → salt + water.
Combustion Reaction
A reaction in which a substance reacts rapidly with oxygen, producing energy (heat and light).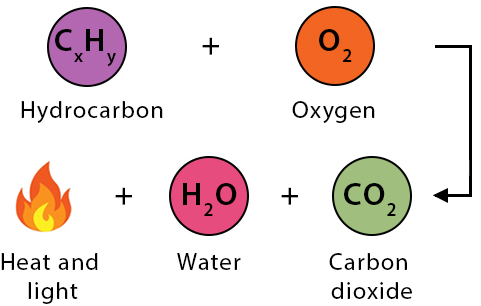
General form: \( \mathrm{Fuel + O_2 \rightarrow CO_2 + H_2O + Energy} \)
Features:
- Always exothermic.
- Involves rapid oxidation.
- Essential in respiration and energy production.
Examples:
- \( \mathrm{CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O + Energy} \)
- \( \mathrm{C + O_2 \rightarrow CO_2 + Energy} \)
- \( \mathrm{2H_2 + O_2 \rightarrow 2H_2O + Energy} \)
Summary Table of Reaction Types
| Reaction Type | General Formula | Energy Change | Example |
|---|---|---|---|
| Synthesis | \( \mathrm{A + B \rightarrow AB} \) | Usually exothermic |  |
| Decomposition | \( \mathrm{AB \rightarrow A + B} \) | Usually endothermic | 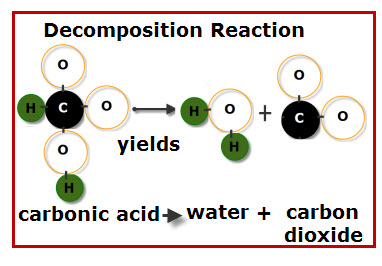 |
| Single Displacement | \( \mathrm{A + BC \rightarrow AC + B} \) | Exothermic | 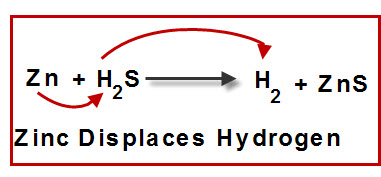 |
| Double Displacement | \( \mathrm{AB + CD \rightarrow AD + CB} \) | Can be exothermic or endothermic | 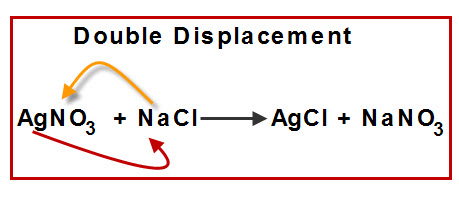 |
| Combustion | \( \mathrm{Fuel + O_2 \rightarrow CO_2 + H_2O + Energy} \) | Exothermic | 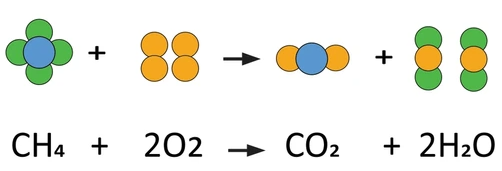 |
Example
When sodium reacts with chlorine gas, sodium chloride is formed. Identify the type of reaction.
▶️ Answer / Explanation
Equation: \( \mathrm{2Na + Cl_2 \rightarrow 2NaCl} \)
Type: Synthesis (Combination).
Explanation: Two elements combine to form a single product.
Example
Barium chloride reacts with sodium sulfate to form a white precipitate of barium sulfate. Identify the reaction type and reason.
▶️ Answer / Explanation
Equation: \( \mathrm{BaCl_2 + Na_2SO_4 \rightarrow BaSO_4↓ + 2NaCl} \)
Type: Double Displacement (precipitation reaction).
Explanation: Exchange of ions occurs, forming insoluble barium sulfate.
Example
Explain why combustion reactions are always exothermic and describe the bond energy changes involved.
▶️ Answer / Explanation
Step 1: Combustion involves burning a fuel in oxygen to form oxides.
Step 2: Bonds in the fuel (C–H, C–C) and oxygen (O=O) are broken → energy absorbed.
Step 3: Stronger bonds (C=O and O–H) form in CO₂ and H₂O → energy released.
Step 4: Since released energy > absorbed energy, the net effect is exothermic.
Final Answer: Combustion is exothermic because more energy is released during bond formation than is needed for bond breaking.
