IB MYP 4-5 Chemistry -Uses and risks of radioactivity- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Uses and risks of radioactivity- Study Notes
Key Concepts
- Uses and Risks of Radioactivity
- Nuclear Waste Disposal and Safety Measures
Uses and Risks of Radioactivity
Uses and Risks of Radioactivity
Radioactivity is the spontaneous emission of radiation (α, β, or γ) from unstable atomic nuclei. While radioactivity has many beneficial applications in science and technology, it also poses serious health and environmental risks if not properly managed.
Useful Applications of Radioactivity
Radioisotopes (unstable isotopes that emit radiation) are widely used in various fields such as medicine, industry, agriculture, and energy.
![]()
A. Medical Applications
- Diagnosis (Imaging): Radioactive tracers such as \( \mathrm{^{99m}Tc} \) (technetium-99m) are injected into the body to monitor organ function and detect tumors or blockages using gamma cameras.
- Treatment (Radiotherapy): High-energy gamma rays from cobalt-60 or cesium-137 are used to destroy cancerous cells.
- Sterilization: Gamma radiation sterilizes medical equipment and surgical instruments without using heat.
Example Isotopes:
| Isotope | Use | Type of Radiation |
|---|---|---|
| \( \mathrm{^{99m}Tc} \) | Diagnostic imaging (tracer) | γ |
| \( \mathrm{^{60}Co} \) | Cancer treatment (radiotherapy) | γ |
| \( \mathrm{^{131}I} \) | Thyroid gland studies and treatment | β and γ |
B. Industrial Applications
- Thickness control: Beta emitters (e.g., \( \mathrm{^{14}C} \), \( \mathrm{^{90}Sr} \)) are used to monitor the thickness of materials like paper, plastic, or metal sheets in manufacturing.
- Leak detection: Radioactive tracers detect pipeline leaks by tracking the flow of emitted radiation.
- Power generation: Uranium-235 and plutonium-239 are used as fuel in nuclear reactors to generate electricity through controlled fission.
C. Agricultural and Environmental Uses
- Food irradiation: Gamma rays kill bacteria and pests to preserve food for longer periods.
- Fertilizer efficiency: Radioisotopes like \( \mathrm{^{32}P} \) track nutrient absorption in plants.
- Pollution tracing: Radioactive tracers help track the movement of pollutants in rivers and oceans.
D. Scientific and Archaeological Uses
- Radiocarbon dating: \( \mathrm{^{14}C} \) is used to estimate the age of ancient organic materials (fossils, bones, wood).
- Tracer studies: Used in chemistry and biology to follow the path of elements in reactions or ecosystems.
Risks and Dangers of Radioactivity
While radiation has valuable uses, exposure beyond safe levels can cause serious harm to living organisms and the environment.
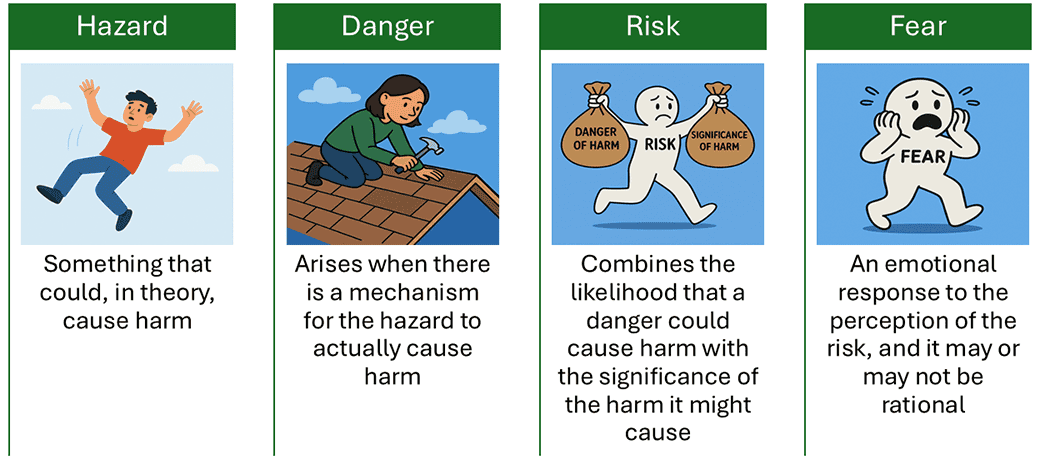
A. Biological Risks
- Cell damage: Ionizing radiation can damage DNA and cell structures.
- Health effects: High doses can cause radiation sickness, burns, cancer, or genetic mutations.
- Internal contamination: Inhalation or ingestion of radioactive particles (e.g., radon gas, iodine-131) is especially dangerous.
B. Environmental Risks
- Radioactive waste: Byproducts from reactors and medical sources remain hazardous for thousands of years.
- Nuclear accidents: Events like Chernobyl (1986) and Fukushima (2011) released large amounts of radiation into the environment.
- Bioaccumulation: Radioactive isotopes can enter food chains, affecting ecosystems and human health.
C. Safety Precautions
![]()
- Minimize exposure time and increase distance from radiation sources.
- Use shielding materials (lead, concrete, water) to block radiation.
- Wear protective clothing and monitor exposure with dosimeters.
- Store radioactive materials in secure, shielded containers.
Summary Table: Uses and Risks of Radioactivity
| Category | Main Uses | Major Risks |
|---|---|---|
| Medicine | Diagnosis, cancer treatment, sterilization | Overexposure damages healthy tissue |
| Industry | Thickness control, leak detection, power generation | Worker exposure, radioactive waste |
| Agriculture | Food preservation, soil nutrient studies | Possible contamination of food |
| Environment | Pollution tracing, dating fossils | Ecosystem damage, bioaccumulation |
Example
How is technetium-99m used in medicine, and why is it suitable for this purpose?
▶️ Answer / Explanation
Step 1: Technetium-99m emits gamma rays, which can pass through the body and be detected by cameras.
Step 2: It has a short half-life (~6 hours), minimizing long-term radiation exposure.
Final Answer: \( \mathrm{^{99m}Tc} \) is used as a medical tracer because it emits detectable gamma rays and decays quickly, reducing patient risk.
Example
Explain why gamma rays are preferred over alpha or beta radiation for cancer treatment.
▶️ Answer / Explanation
Step 1: Gamma rays have very high penetrating power and can reach deep tumors inside the body.
Step 2: Alpha and beta particles cannot penetrate deeply enough and would damage only surface tissues.
Final Answer: Gamma rays are used for radiotherapy because they can target and destroy deep-seated cancer cells without surgery.
Example
Discuss the advantages and disadvantages of using nuclear power for electricity generation.
▶️ Answer / Explanation
Step 1: Advantages: Produces huge amounts of energy with very little fuel; no greenhouse gases during operation; reliable energy source.
Step 2: Disadvantages: Produces radioactive waste; potential for nuclear accidents; high cost and long construction times.
Step 3: Balance: While nuclear power reduces fossil fuel use, strict safety and waste management systems are essential.
Final Answer: Nuclear energy is efficient and low-carbon but poses major safety and waste challenges that must be managed responsibly.
Nuclear Waste Disposal and Safety Measures
Nuclear Waste Disposal and Safety Measures
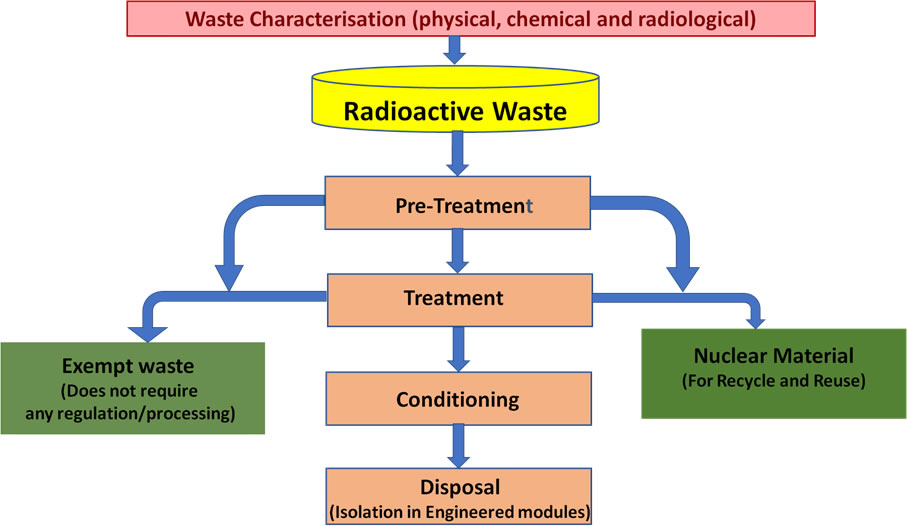
Nuclear waste (or radioactive waste) refers to materials that contain unstable isotopes and continue to emit radiation after being used in nuclear reactors, medicine, or research. Safe disposal and management are essential to protect people and the environment from harmful radiation exposure.
Types of Nuclear Waste
Nuclear waste is categorized based on its radioactivity, heat generation, and lifetime of radioactive decay.

| Category | Description | Examples | Handling / Disposal |
|---|---|---|---|
| Low-Level Waste (LLW) | Contains small amounts of short-lived radioisotopes. | Protective clothing, tools, lab materials. | Buried in shallow landfills after decay storage. |
| Intermediate-Level Waste (ILW) | Contains higher radioactivity; may require shielding. | Resins, reactor components, chemical sludge. | Encased in concrete or bitumen and stored underground. |
| High-Level Waste (HLW) | Extremely radioactive, generates large amounts of heat. | Spent fuel rods, nuclear reactor waste. | Stored in deep geological repositories for thousands of years. |
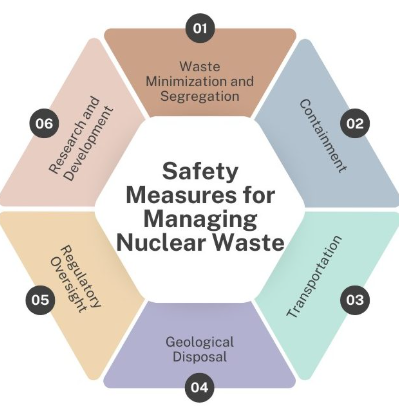
Methods of Nuclear Waste Disposal

- Decay Storage: Low-level waste is stored safely until its radioactivity decreases to safe levels.
- Deep Geological Disposal: High-level waste is sealed in corrosion-resistant containers and buried deep underground (typically 300–1000 m) in stable rock formations.
- Immobilization: Liquid waste is converted into solid glass-like material (vitrification) to prevent leakage.
- Containment and Shielding: Waste is enclosed in multiple protective barriers — steel drums, concrete, or lead — to prevent radiation escape.
- Reprocessing and Recycling: Some nuclear fuels (like uranium or plutonium) are chemically reprocessed and reused in reactors, reducing total waste volume.
Environmental and Safety Concerns
- Radioactive Leakage: Improper containment can contaminate soil, groundwater, and ecosystems.
- Long Half-Lives: Some isotopes (e.g., plutonium-239) remain hazardous for tens of thousands of years.
- Accidental Exposure: Mishandling or accidents during transport or storage can cause severe radiation damage.
- Nuclear Accidents: Catastrophic failures (e.g., Chernobyl, Fukushima) highlight the need for strict safety controls.
Safety Measures for Handling and Storage
- Shielding: Use of dense materials like lead, concrete, or water to absorb radiation.
- Distance and Time Control: Minimizing exposure time and maintaining safe distance from radioactive materials.
- Secure Containers: Double-sealed, corrosion-resistant containers to prevent leaks.
- Monitoring and Detection: Continuous monitoring using Geiger counters and radiation detectors.
- Protective Gear: Workers use lead-lined clothing, gloves, and dosimeters.
- Site Selection: Geological stability, low water flow, and minimal earthquake risk are required for waste storage sites.
Long-Term Waste Management Strategies
- Geological Repositories: Deep underground facilities (e.g., Finland’s Onkalo repository) provide permanent isolation for high-level waste.
- Recycling of Spent Fuel: Recovering uranium and plutonium reduces the volume of dangerous waste.
- Transmutation Research: Advanced reactors or accelerators can convert long-lived isotopes into shorter-lived ones, reducing hazard time.
Nuclear Waste Management Overview
| Waste Type | Example | Disposal Method | Storage Duration |
|---|---|---|---|
| Low-Level Waste (LLW) | Gloves, clothing | Surface burial after short storage | Up to 100 years |
| Intermediate-Level Waste (ILW) | Reactor parts | Encased and stored underground | Hundreds to thousands of years |
| High-Level Waste (HLW) | Spent fuel rods | Deep geological repository | Thousands to millions of years |
Example
Why must high-level nuclear waste be stored deep underground?
▶️ Answer / Explanation
Step 1: High-level waste emits strong radiation and heat for thousands of years.
Step 2: Storing it deep underground prevents radiation leakage and environmental contamination.
Final Answer: Deep geological storage ensures isolation of radioactive waste from humans and ecosystems for long periods.
Example
Explain why vitrification is preferred for long-term storage of liquid radioactive waste.
▶️ Answer / Explanation
Step 1: Liquid waste can leak or spread easily if containers fail.
Step 2: In vitrification, waste is mixed with glass-forming materials and melted into a stable, solid form.
Step 3: This solid is durable, non-reactive, and resists leakage.
Final Answer: Vitrification prevents radioactive leaks and provides stable, long-term containment.
Example
Discuss how the reprocessing of spent nuclear fuel reduces both environmental and economic impacts of nuclear waste disposal.
▶️ Answer / Explanation
Step 1: Spent fuel rods contain unused uranium and plutonium that can be recovered.
Step 2: Reprocessing separates these usable isotopes for reuse, reducing the volume and radioactivity of remaining waste.
Step 3: This decreases the amount of waste requiring long-term storage and saves cost on new fuel production.
Final Answer: Reprocessing minimizes waste volume, recycles valuable materials, and lowers both environmental hazard and nuclear fuel expenses.
