IB MYP 4-5 Chemistry -Uses of metals and alloys- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Uses of metals and alloys- Study Notes
Key Concepts
- Uses of Metals and Alloys
- Properties of Metals and Alloys
Uses of Metals and Alloys
Uses of Metals and Alloys
Metals and alloys are essential materials used in everyday life due to their wide range of physical and chemical properties such as strength, conductivity, malleability, ductility, and resistance to corrosion.
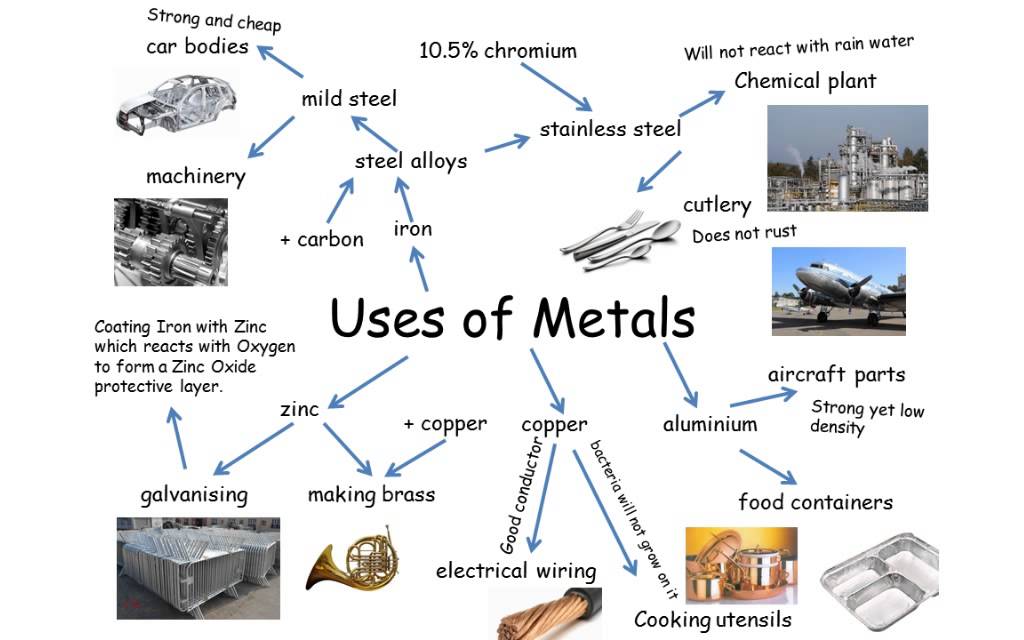
The use of a metal or alloy depends on its specific properties — for example, light metals like aluminium are used for aircraft, while hard alloys like steel are used for construction.
Common Uses of Metals
| Metal | Key Properties | Common Uses |
|---|---|---|
| Iron (Fe) | Strong, malleable, magnetic, conducts heat and electricity | Construction, machinery, bridges, tools |
| Copper (Cu) | Excellent conductor, malleable, corrosion-resistant | Electrical wiring, water pipes, cookware |
| Aluminium (Al) | Lightweight, corrosion-resistant, good conductor | Aircraft bodies, packaging (foil, cans), window frames |
| Zinc (Zn) | Moderately reactive, forms protective oxide layer | Galvanizing iron, making batteries, alloys like brass |
| Gold (Au) | Highly malleable, ductile, corrosion-resistant, good conductor | Jewellery, electrical connectors, decorative applications |
| Silver (Ag) | Best electrical conductor, reflective, antimicrobial | Jewellery, mirrors, electronics, medical tools |
| Lead (Pb) | Dense, soft, corrosion-resistant, low melting point | Car batteries, radiation shielding (X-ray protection) |
Alloys — Definition and Importance
An alloy is a mixture of two or more elements, where at least one is a metal. Alloys are designed to improve properties like strength, hardness, resistance to corrosion, or appearance.
![]()
Alloys are usually stronger and more durable than pure metals due to the disruption of regular atomic arrangement, which makes it harder for layers of atoms to slide over each other.
Common Alloys, Composition, and Uses
| Alloy | Main Components | Improved Property | Common Uses |
|---|---|---|---|
| Steel | Iron + Carbon | Stronger, less brittle than pure iron | Construction, machinery, tools |
| Stainless Steel | Iron + Chromium + Nickel | Corrosion-resistant and shiny | Kitchenware, surgical tools, architecture |
| Brass | Copper + Zinc | Harder and more corrosion-resistant than copper | Musical instruments, door handles, plumbing |
| Bronze | Copper + Tin | Hard, corrosion-resistant, and durable | Statues, coins, ship propellers |
| Duralumin | Aluminium + Copper + Magnesium + Manganese | Light, strong, and resistant to corrosion | Aircraft and automobile parts |
| Solder | Lead + Tin | Low melting point, good for joining metals | Electrical connections, plumbing joints |
| Nichrome | Nickel + Chromium | High melting point, resistant to oxidation | Heating elements (toasters, hair dryers) |
Advantages of Using Alloys over Pure Metals
- Stronger and harder than pure metals.
- More resistant to corrosion and oxidation.
- Can be made to have specific properties (e.g., magnetic, lightweight, conductive).
- Often have lower melting points for easier manufacturing.
- Used to improve both functional and aesthetic qualities.
Example
Why is copper used for electrical wiring instead of iron?
▶️ Answer / Explanation
Step 1: Copper has very low electrical resistance and is a better conductor than iron.
Step 2: Copper also resists corrosion and is easily drawn into wires.
Final Answer: Copper is preferred because it conducts electricity efficiently and does not rust easily.
Example
Why is steel used for construction instead of pure iron?
▶️ Answer / Explanation
Step 1: Pure iron is soft and easily bends under stress.
Step 2: Adding carbon forms steel, which is much harder and stronger.
Step 3: Steel retains some flexibility and is resistant to deformation.
Final Answer: Steel combines strength and durability, making it ideal for buildings and bridges.
Example
Compare and explain why aluminium is used in aircraft construction while steel is used in bridges, even though both are strong metals.
▶️ Answer / Explanation
Step 1: Aluminium is lightweight (density ≈ 2.7 g/cm³) while steel is heavy (≈ 7.8 g/cm³).
Step 2: Aircraft require materials with high strength-to-weight ratio to reduce fuel consumption.
Step 3: Aluminium and its alloys resist corrosion from air and moisture.
Step 4: Steel is stronger and more economical, ideal for heavy load-bearing structures.
Final Answer: Aluminium is used where weight reduction is critical, while steel is used where maximum strength and cost efficiency are needed.
Properties of Metals and Alloys
Properties of Metals and Alloys
Metals are elements that are typically hard, shiny, malleable, ductile, and good conductors of heat and electricity. An alloy is a mixture of two or more elements, where at least one is a metal, designed to improve or modify the original properties of the pure metal.
Alloys often have superior properties to pure metals — they can be stronger, harder, and more resistant to corrosion, depending on their composition and structure.
General Properties of Metals
Metals share a number of physical and chemical properties that make them useful in various applications.

| Property | Description | Examples / Notes |
|---|---|---|
| Lustre | Metals have a shiny, reflective surface when polished. | Gold, silver, and aluminium reflect light well. |
| Malleability | Can be hammered or rolled into thin sheets without breaking. | Aluminium foil, iron sheets |
| Ductility | Can be drawn into wires. | Copper and gold are highly ductile. |
| Conductivity | Good conductors of heat and electricity due to free-moving electrons. | Copper wires, aluminium cables |
| High Melting and Boiling Points | Strong metallic bonds require large energy to break. | Tungsten has one of the highest melting points (~3400°C). |
| Sonority | Produce a ringing sound when struck. | Bells, cymbals |
| Density and Strength | Most metals are dense and can withstand heavy loads. | Iron and lead are dense metals; aluminium is light but strong. |
| Reactivity | Different metals have different tendencies to react with air, water, or acids. | Potassium reacts vigorously with water; gold does not react. |
Properties of Alloys Compared to Pure Metals
Alloys often exhibit improved or modified properties compared to their base metals due to the disruption of the regular metallic lattice.
| Property | Pure Metal | Alloy | Example / Note |
|---|---|---|---|
| Strength | Soft and can deform easily. | Stronger and more rigid. | Steel (Fe + C) is stronger than pure iron. |
| Hardness | Atoms slide easily over each other. | Harder — atoms of different sizes block layers from sliding. | Brass and bronze are harder than copper. |
| Corrosion Resistance | May react with air or moisture. | Often more resistant to oxidation and rusting. | Stainless steel resists rust due to chromium. |
| Malleability & Ductility | Usually high — can be shaped easily. | Reduced slightly due to irregular structure. | Brass is less malleable than pure copper. |
| Electrical Conductivity | Excellent conductor due to free electrons. | Slightly lower due to atomic distortion. | Copper conducts better than brass. |
| Melting Point | Definite, sharp melting point. | Lower or ranges over temperatures. | Solder melts at lower temperature than its components. |
Reasons Why Alloys Have Different Properties
- Different-sized atoms disrupt the regular arrangement of metal ions.
- This makes it more difficult for atomic layers to slide — increasing strength and hardness.
- Presence of other elements can prevent oxidation or corrosion.
- Electrical and thermal conductivities may decrease due to irregular structure.
Example
Why are metals good conductors of electricity?
▶️ Answer / Explanation
Step 1: Metals contain free (delocalized) electrons in a “sea of electrons.”
Step 2: These electrons move easily through the structure when voltage is applied.
Final Answer: Electrical conductivity in metals is due to free-moving delocalized electrons that carry charge efficiently.
Example
Why is steel harder and stronger than pure iron?
▶️ Answer / Explanation
Step 1: In pure iron, atoms are uniform, allowing layers to slide easily.
Step 2: In steel, small carbon atoms fit in gaps between iron atoms.
Step 3: This prevents atomic layers from moving, increasing hardness and strength.
Final Answer: The addition of carbon disrupts the lattice, making steel stronger and less malleable.
Example
Compare the electrical conductivity and strength of pure copper and brass, and explain why brass is used for door handles rather than for electrical wires.
▶️ Answer / Explanation
Step 1: Pure copper conducts electricity better because it has a uniform atomic lattice.
Step 2: Brass (copper + zinc) has atoms of different sizes, reducing electron mobility but increasing hardness.
Step 3: Door handles need strength and corrosion resistance, not conductivity.
Final Answer: Brass is harder and more corrosion-resistant, making it suitable for mechanical use, while copper is better for electrical wiring.
