IB MYP 4-5 Chemistry -Water purification and treatment- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Water purification and treatment- Study Notes
Key Concepts
- Water Purification and Treatment
- Wastewater Treatment and Water Pollution Control
Water Purification and Treatment
Water Purification and Treatment
Water purification is the process of removing physical, chemical, and biological impurities from water to make it safe for human use. It ensures that drinking water is clean, clear, and free of harmful microorganisms and toxic substances.
Water treatment refers to the set of processes used in water treatment plants to convert raw water (from rivers, lakes, or groundwater) into potable (drinking) water.
Why Water Purification Is Necessary
Natural water sources often contain impurities such as:
- Suspended solids: sand, silt, and clay particles
- Microorganisms: bacteria, viruses, and protozoa
- Dissolved salts: calcium, magnesium, nitrates, and chlorides
- Chemical pollutants: pesticides, industrial effluents, and heavy metals
Goal: To remove these contaminants to produce clean, safe, and odor-free water.
Main Stages of Water Treatment
Municipal water treatment usually involves several stages:
![]()
| Stage | Process | Purpose |
|---|---|---|
| 1. Screening | Large objects like leaves and plastic are removed using metal screens. | Prevents clogging of treatment equipment. |
| 2. Sedimentation | Water is allowed to stand so heavier particles settle at the bottom. | Removes suspended solids. |
| 3. Coagulation and Flocculation | Chemicals like alum (\( \mathrm{KAl(SO_4)_2 \cdot 12H_2O} \)) are added to form sticky flocs that trap fine particles. | Removes colloidal particles and dirt. |
| 4. Filtration | Water passes through layers of sand, gravel, and charcoal. | Removes fine particles and microorganisms. |
| 5. Disinfection | Chlorine, ozone, or UV light is used to kill harmful microorganisms. | Makes water microbiologically safe for drinking. |
Common Methods of Water Purification
(a) Filtration
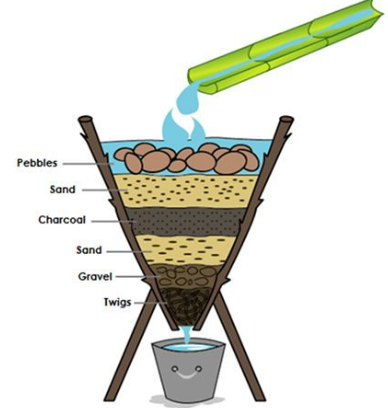
- Physical removal of impurities using porous materials (sand, ceramic, activated carbon).
- Activated carbon filters absorb organic compounds and remove odors.
(b) Chlorination
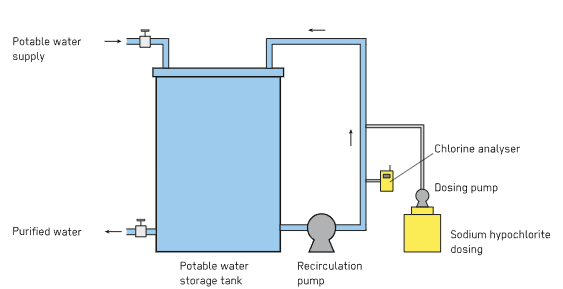
- Adding chlorine or bleaching powder (\( \mathrm{Ca(OCl)_2} \)) to water.
- Reaction: \( \mathrm{Cl_2 + H_2O \rightarrow HCl + HOCl} \)
- Hypochlorous acid (\( \mathrm{HOCl} \)) kills bacteria and viruses.
(c) Boiling
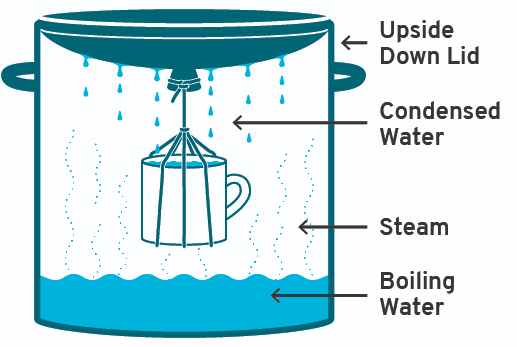
- Boiling water for 10–15 minutes kills microorganisms.
- Suitable for household use but not for large-scale treatment.
(d) Ozonation
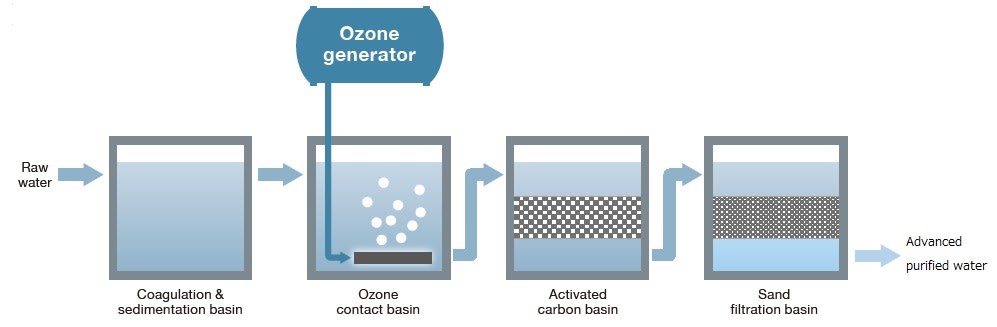
- Ozone (\( \mathrm{O_3} \)) is bubbled through water to kill bacteria and oxidize pollutants.
- Leaves no chemical residue, making it environmentally friendly.
(e) Reverse Osmosis (RO)
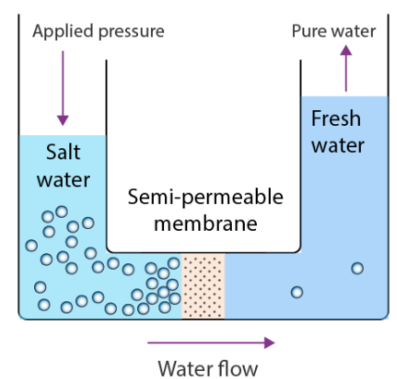
- Water is forced through a semi-permeable membrane under pressure.
- Removes salts, ions, and microbes — produces very pure water.
- Equation: \( \mathrm{H_2O_{impure} \xrightarrow{pressure} H_2O_{pure}} \)
Hard Water and Its Treatment
Hard water contains dissolved calcium and magnesium ions (\( \mathrm{Ca^{2+},\ Mg^{2+}} \)) that prevent soap from lathering.
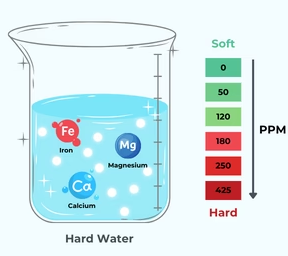
Temporary Hardness: Caused by bicarbonates of calcium and magnesium.
Removal by Boiling: \( \mathrm{Ca(HCO_3)_2 \rightarrow CaCO_3 \downarrow + H_2O + CO_2} \)
Permanent Hardness: Caused by sulfates and chlorides of calcium and magnesium.
Removed by Ion-Exchange (Zeolite) Method:
\( \mathrm{Na_2Z + Ca^{2+} \rightarrow CaZ + 2Na^+} \)
Desalination (Purification of Seawater)
Desalination removes salt and other minerals from seawater to make it drinkable. Common methods:
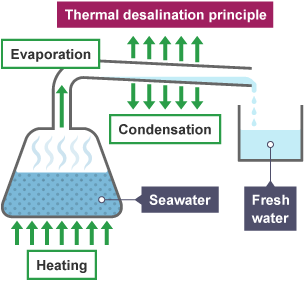
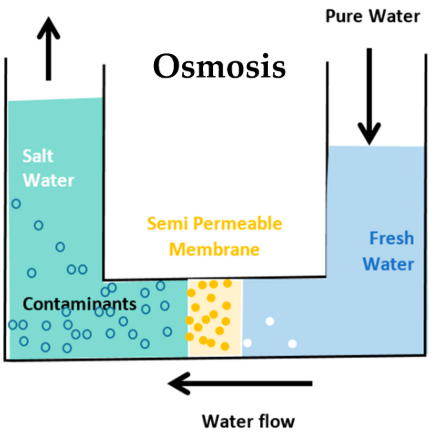
- Distillation: Boiling and condensing vapor to get pure water.
- Reverse Osmosis (RO): Pressurizing seawater through a membrane that allows only water molecules to pass.
Example
Why is chlorine added to water during purification?
▶️ Answer / Explanation
Step 1: Chlorine reacts with water to form hypochlorous acid (\( \mathrm{HOCl} \)).
Step 2: \( \mathrm{Cl_2 + H_2O \rightarrow HCl + HOCl} \)
Step 3: \( \mathrm{HOCl} \) kills bacteria and other pathogens.
Final Answer: Chlorine disinfects water by forming \( \mathrm{HOCl} \), which destroys harmful microorganisms.
Example
Explain how reverse osmosis (RO) removes dissolved salts from seawater.
▶️ Answer / Explanation
Step 1: In RO, pressure is applied to seawater to force it through a semi-permeable membrane.
Step 2: Water molecules pass through, but salts and impurities are left behind.
Step 3: The process is the reverse of natural osmosis.
Final Answer: RO removes dissolved salts by forcing pure water through a membrane while blocking ions and pollutants.
Example
Why is excessive chlorination of drinking water harmful, and how can it be controlled?
▶️ Answer / Explanation
Step 1: Too much chlorine can react with organic matter to form harmful chlorinated compounds like trihalomethanes (THMs).
Step 2: These compounds may be carcinogenic or cause taste and odor issues.
Step 3: Controlled chlorination (1–2 mg/L) ensures disinfection without harmful side effects.
Final Answer: Excess chlorination is harmful; maintaining correct chlorine levels ensures safe and effective water purification.
Wastewater Treatment and Water Pollution Control
Wastewater Treatment and Water Pollution Control
Wastewater is water that has been used and contains impurities from homes, industries, or agriculture. It includes domestic sewage, industrial effluents, and agricultural runoff. Wastewater treatment is the process of cleaning used water so it can be safely returned to the environment or reused.
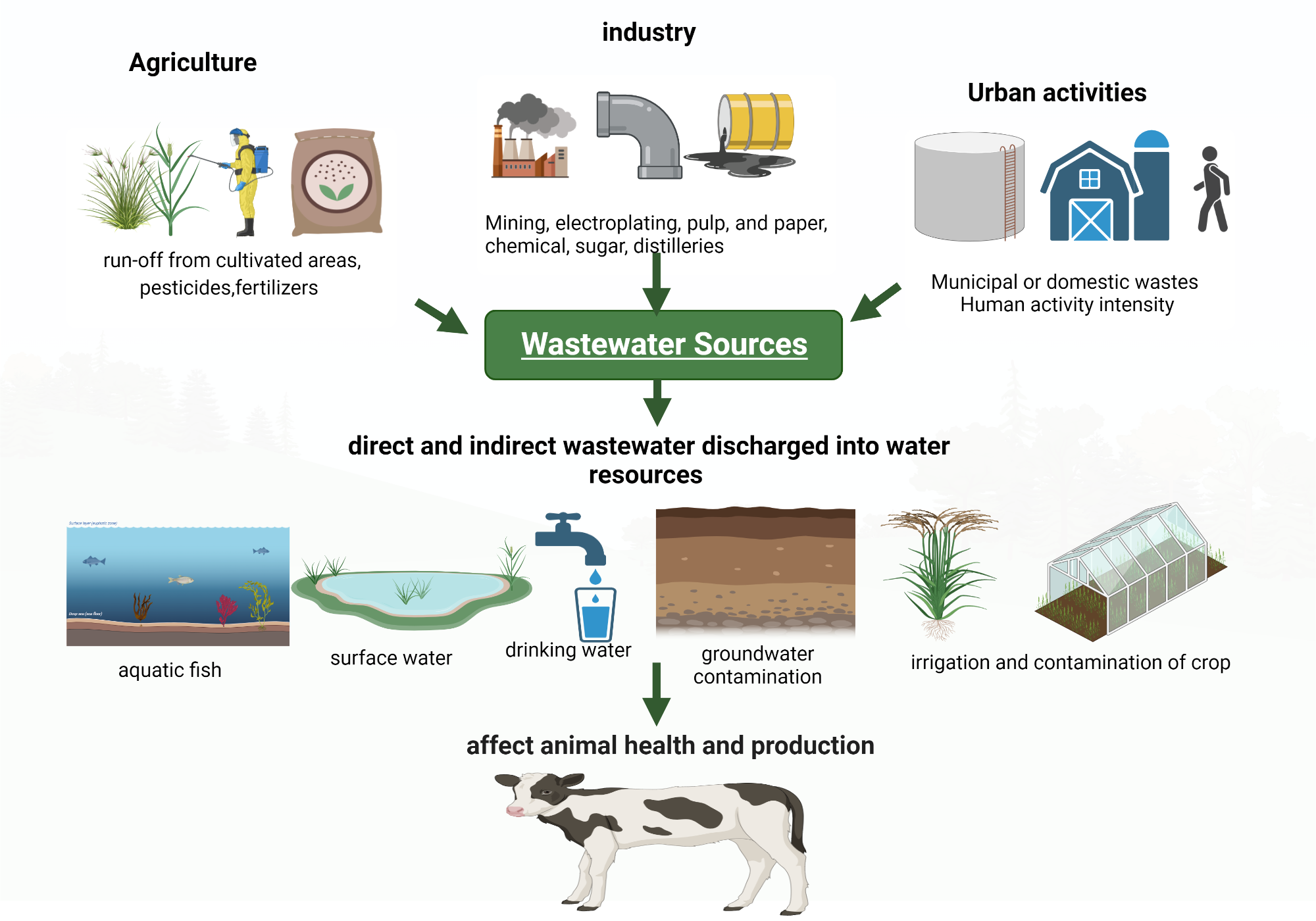
Sources of Wastewater
- Domestic: From households — sinks, toilets, washing, cooking.
- Industrial: From factories — chemicals, heavy metals, dyes, oils.
- Agricultural: From irrigation runoff — fertilizers, pesticides, animal waste.
- Stormwater: Rainwater runoff from roads and urban areas carrying dirt and oil.
Why Wastewater Treatment Is Necessary
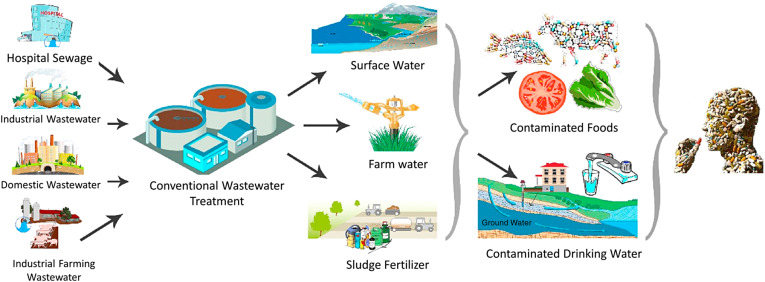
- Prevents the spread of diseases caused by pathogens in sewage.
- Removes toxic substances harmful to aquatic life and humans.
- Prevents oxygen depletion in rivers and lakes.
- Allows safe reuse of treated water for irrigation and industrial cooling.
Stages of Wastewater Treatment
Modern sewage treatment plants use a series of physical, biological, and chemical processes to purify wastewater.
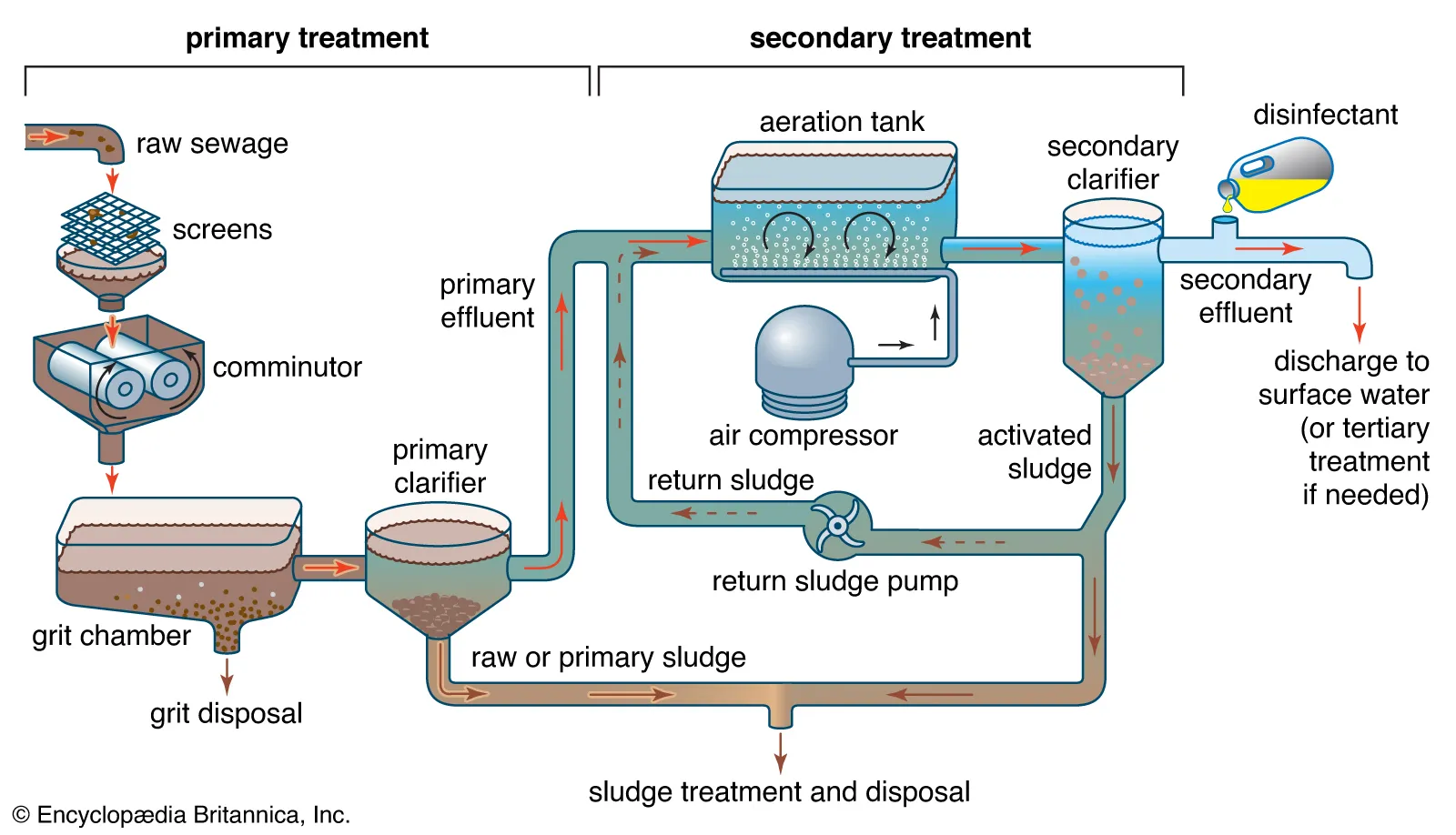
| Stage | Process | Purpose |
|---|---|---|
| 1. Preliminary Treatment | Screens and grit chambers remove large solids (plastic, rags, sand). | Prevents clogging of equipment. |
| 2. Primary Treatment | Sedimentation tanks allow suspended solids to settle as sludge. | Removes 50–60% of suspended solids. |
| 3. Secondary Treatment (Biological) | Aerobic bacteria decompose organic matter in aeration tanks. | Removes biodegradable organic waste. |
| 4. Tertiary Treatment (Chemical/Advanced) | Chemical coagulation, filtration, and disinfection with chlorine or ozone. | Removes pathogens, nitrates, phosphates, and final impurities. |
| 5. Sludge Treatment | Sludge from sedimentation is treated anaerobically to produce biogas and fertilizer. | Energy recovery and safe disposal of solid waste. |
Major Pollutants in Wastewater
| Type of Pollutant | Examples | Effect |
|---|---|---|
| Organic Waste | Food waste, sewage, plant matter | Depletes oxygen → fish death |
| Pathogens | Bacteria, viruses, protozoa | Causes waterborne diseases |
| Chemicals | Detergents, pesticides, acids | Toxic to aquatic organisms |
| Heavy Metals | Lead, mercury, cadmium | Bioaccumulation in food chains |
Water Pollution — Causes and Effects
Main Causes: 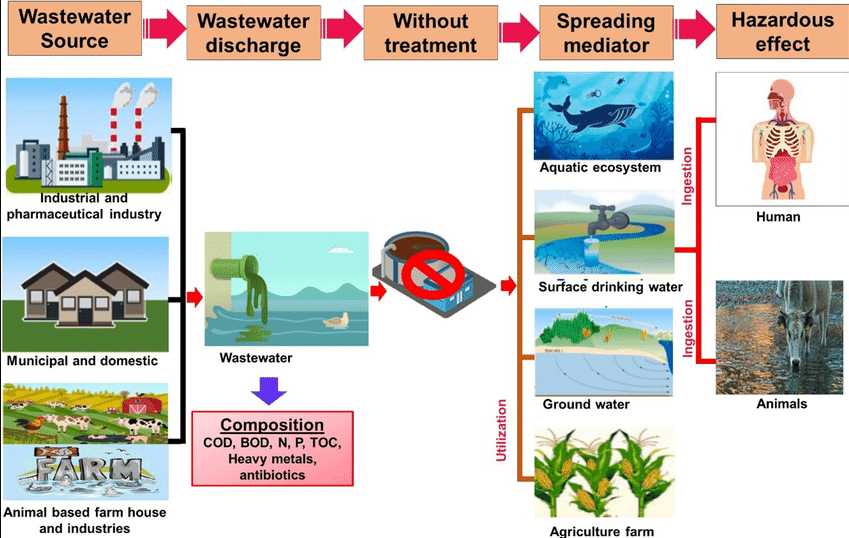
- Discharge of untreated sewage and industrial waste into rivers and seas.
- Agricultural runoff containing fertilizers (nitrates, phosphates) and pesticides.
- Oil spills and dumping of plastics and chemicals.
- Thermal pollution from power plants (hot water discharge).
Effects:
- Depletion of dissolved oxygen (DO) → aquatic life death.
- Eutrophication — nutrient overload causing algal blooms.
- Spread of diseases like cholera, typhoid, and dysentery.
- Contamination of groundwater affecting drinking water quality.
Equation (Eutrophication Example):
\( \mathrm{PO_4^{3-} + NO_3^- \rightarrow Algal\ growth \rightarrow Oxygen\ depletion} \)
Water Pollution Control Measures
- Treat sewage before discharge: All wastewater should pass through treatment plants.
- Industrial effluent control: Use neutralization, filtration, or precipitation before release.
- Reduce fertilizer and pesticide use: Adopt organic farming and controlled irrigation.
- Oil spill management: Use absorbent booms or chemical dispersants.
- Public awareness and legislation: Enforce strict pollution control laws and education campaigns.
Example
What is the purpose of secondary treatment in wastewater purification?
▶️ Answer / Explanation
Step 1: Secondary treatment uses microorganisms to break down organic matter.
Step 2: It converts waste into harmless carbon dioxide, water, and biomass.
Step 3: Reduces Biological Oxygen Demand (BOD).
Final Answer: Secondary treatment biologically removes organic pollutants and reduces BOD levels.
Example
Explain how eutrophication affects aquatic ecosystems.
▶️ Answer / Explanation
Step 1: Fertilizers containing nitrates and phosphates enter water bodies.
Step 2: Excess nutrients cause overgrowth of algae (algal bloom).
Step 3: When algae die, decomposition uses up oxygen.
Step 4: Oxygen depletion kills fish and aquatic life.
Final Answer: Eutrophication leads to oxygen depletion, killing aquatic organisms and disrupting ecosystems.
Example
Suggest and explain three sustainable ways to reduce water pollution in urban areas.
▶️ Answer / Explanation
Step 1: Build efficient sewage treatment plants to process all domestic wastewater.
Step 2: Use green infrastructure (rain gardens, permeable pavements) to filter stormwater naturally.
Step 3: Implement strict industrial waste disposal regulations and promote recycling.
Final Answer: Sustainable solutions include better treatment systems, eco-friendly city designs, and enforcement of pollution control laws.
