IB MYP 4-5 Chemistry -What happens in a chemical reaction- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -What happens in a chemical reaction- Study Notes
Key Concepts
- What Happens in a Chemical Reaction
What Happens in a Chemical Reaction
What Happens in a Chemical Reaction
A chemical reaction is a process in which one or more substances (reactants) are converted into new substances (products) with different chemical properties. During a chemical reaction, the bonds between atoms are broken in the reactants and new bonds are formed in the products.
Key Concept: The total number of atoms of each element is conserved during a chemical reaction matter is neither created nor destroyed.
What Actually Happens During a Reaction 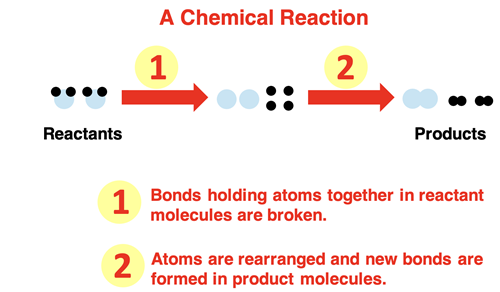
- 1. Breaking of Bonds: Energy is absorbed to break the bonds in the reactant molecules.
- 2. Rearrangement of Atoms: Atoms rearrange to form new combinations.
- 3. Formation of New Bonds: Energy is released when new bonds form in the products.
Energy Changes:
- Endothermic reactions: Energy is absorbed from the surroundings (temperature drops).
- Exothermic reactions: Energy is released to the surroundings (temperature rises).
Equation Representation:
\( \mathrm{Reactants \rightarrow Products} \)
Example: \( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \)
Evidence of a Chemical Reaction
We can identify that a chemical reaction has occurred through observable changes:
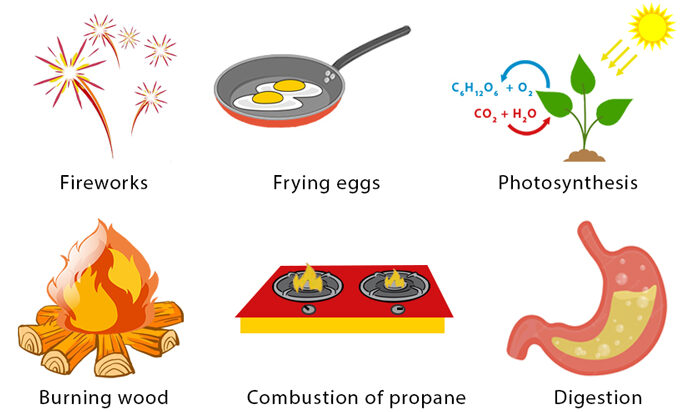
- Color change (e.g., rusting of iron)
- Formation of a gas (bubbles/fizzing)
- Formation of a solid (precipitate)
- Temperature change (heat absorbed or released)
- Light emission or sound produced
Law of Conservation of Mass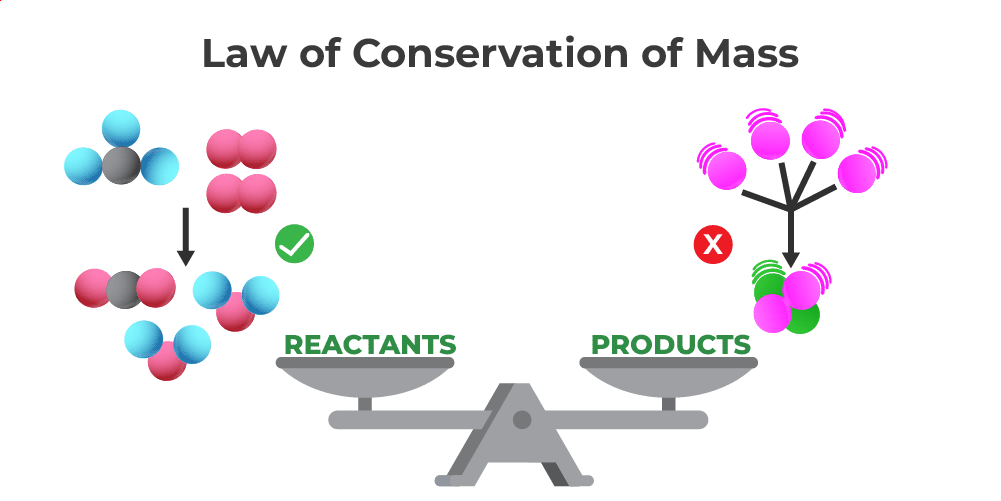
Statement: Mass is neither created nor destroyed in a chemical reaction; it is conserved.
Explanation: Atoms are only rearranged — none are lost or gained. The total mass of reactants = total mass of products.
\( \mathrm{Total\ mass_{reactants} = Total\ mass_{products}} \)
Example: When hydrogen reacts with oxygen to form water, the total mass before and after remains the same:
\( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \)
Types of Energy Changes in Reactions
| Type of Reaction | Energy Flow | Temperature Change | Example |
|---|---|---|---|
| Exothermic | Energy released to surroundings | Increase | \( \mathrm{CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O} \) |
| Endothermic | Energy absorbed from surroundings | Decrease | \( \mathrm{CaCO_3 \rightarrow CaO + CO_2} \) |
Summary of the Process
- Reactant bonds break → energy absorbed.
- Atoms rearrange into new positions.
- New bonds form → energy released.
- Products have different properties from reactants.
- Total mass and number of atoms remain conserved.
Common Types of Chemical Reactions
| Reaction Type | General Form | Example |
|---|---|---|
| Combination (Synthesis) | \( \mathrm{A + B \rightarrow AB} \) | \( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \) |
| Decomposition | \( \mathrm{AB \rightarrow A + B} \) | \( \mathrm{2H_2O \rightarrow 2H_2 + O_2} \) |
| Displacement | \( \mathrm{A + BC \rightarrow AC + B} \) | \( \mathrm{Zn + CuSO_4 \rightarrow ZnSO_4 + Cu} \) |
| Double Displacement | \( \mathrm{AB + CD \rightarrow AD + CB} \) | \( \mathrm{NaCl + AgNO_3 \rightarrow NaNO_3 + AgCl} \) |
Example
When magnesium ribbon burns in air, a white powder is formed. Identify the type of reaction and explain what happens.
▶️ Answer / Explanation
Step 1: Magnesium reacts with oxygen → magnesium oxide forms.
Equation: \( \mathrm{2Mg + O_2 \rightarrow 2MgO} \)
Step 2: Bonds in oxygen break; new Mg–O bonds form → energy released.
Final Answer: Exothermic combination reaction producing a white solid (MgO).
Example
Hydrogen peroxide (\( \mathrm{H_2O_2} \)) decomposes slowly to form water and oxygen. Explain why adding manganese dioxide (\( \mathrm{MnO_2} \)) speeds up the reaction.
▶️ Answer / Explanation
Step 1: Decomposition: \( \mathrm{2H_2O_2 \rightarrow 2H_2O + O_2} \)
Step 2: \( \mathrm{MnO_2} \) acts as a catalyst — lowers activation energy.
Step 3: Reaction occurs faster without being consumed.
Final Answer: The catalyst provides an alternative path with lower energy requirement, speeding up the decomposition.
Example
Explain in detail why energy is both absorbed and released in a chemical reaction, using bond energy concepts.
▶️ Answer / Explanation
Step 1: To start a reaction, reactant bonds must be broken → energy absorbed (endothermic step).
Step 2: When new bonds form in products, energy is released (exothermic step).
Step 3: The overall reaction energy change depends on which process dominates:
- If bond formation energy > bond breaking → exothermic.
- If bond breaking energy > bond formation → endothermic.
Step 4: Example: \( \mathrm{CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O} \) → Breaking C–H and O=O bonds absorbs energy, forming C=O and O–H bonds releases more → net energy released.
Final Answer: Energy absorbed breaks old bonds; energy released forms new ones. The balance of these determines whether the reaction is endothermic or exothermic.
