IB MYP 4-5 Chemistry -Word and balanced chemical equations- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Word and balanced chemical equations- Study Notes
Key Concepts
- Word and Balanced Chemical Equations
Word and Balanced Chemical Equations
Word and Balanced Chemical Equations
A chemical equation is a symbolic representation of a chemical reaction that shows the reactants (starting substances) and products (new substances formed).
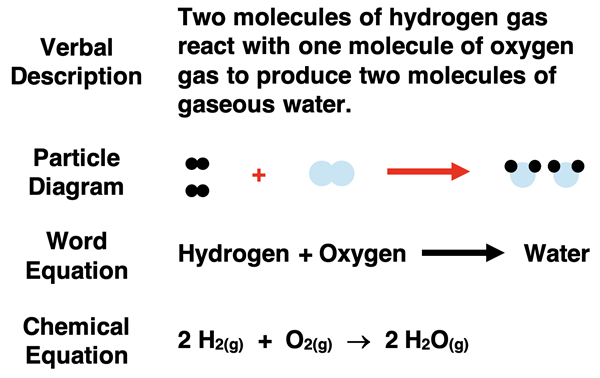
It can be written in two main forms:
- Word Equation – uses words to represent the substances involved.
- Balanced Chemical Equation – uses chemical symbols and formulas to show the same reaction quantitatively.
Word Equations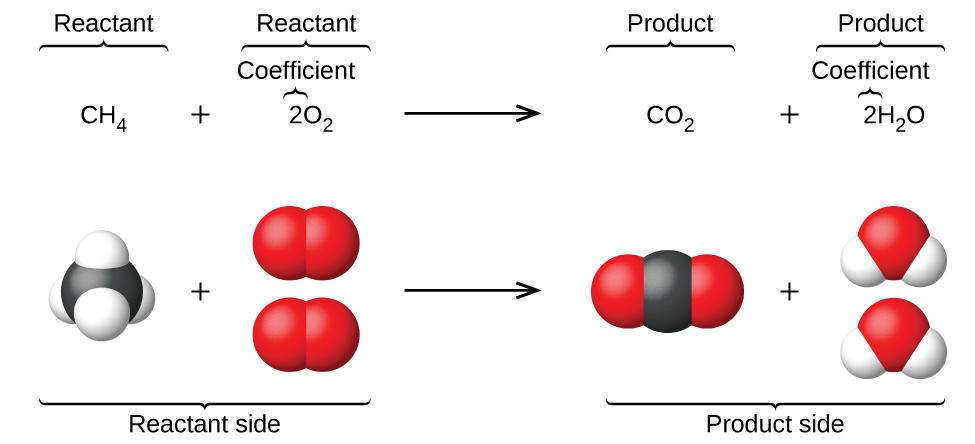
A word equation names the reactants and products in a chemical reaction.
Format:
Reactant + Reactant → Product (+ Product)
Example:
Hydrogen + Oxygen → Water
Explanation: This shows that hydrogen reacts with oxygen to form water, but it doesn’t show the quantities or atomic ratios involved.
Balanced Chemical Equations
A balanced chemical equation uses chemical symbols and formulas to represent the reaction, ensuring that the number of atoms of each element is the same on both sides of the equation.
Example: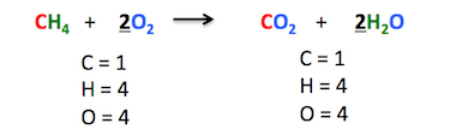
\( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \)
Explanation:
- 2 molecules of hydrogen gas react with 1 molecule of oxygen gas.
- This produces 2 molecules of water.
- The number of hydrogen and oxygen atoms is equal on both sides.
Why Balancing is Important:
- It obeys the Law of Conservation of Mass.
- No atoms are lost or gained; only rearranged.
- Ensures accurate stoichiometric calculations in reactions.
Steps to Write and Balance a Chemical Equation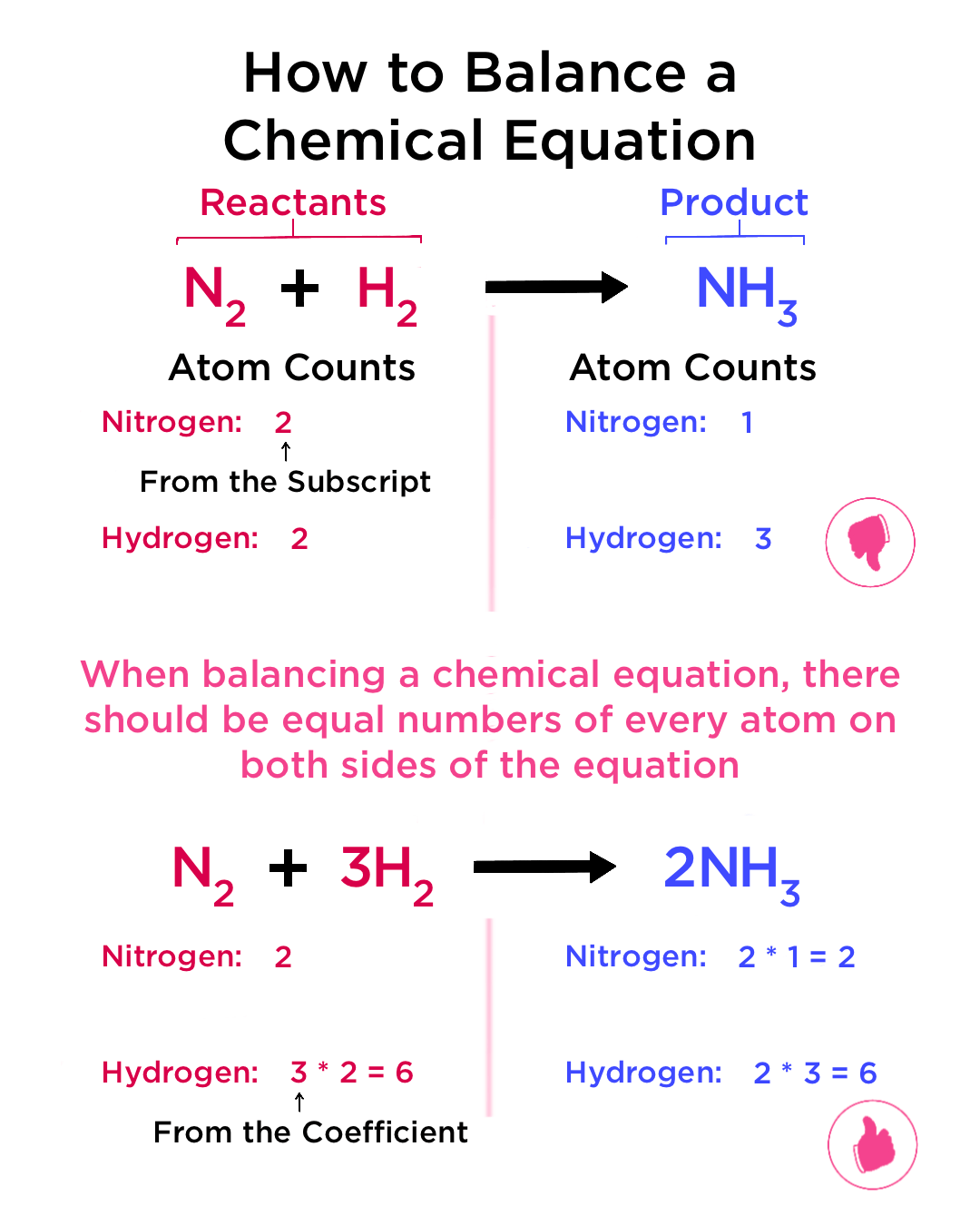
- Step 1: Write the word equation.
- Step 2: Replace names with chemical formulas.
- Step 3: Count atoms of each element on both sides.
- Step 4: Use coefficients (numbers before formulas) to balance atoms.
- Step 5: Check that the equation is balanced — same number of each atom on both sides.
- Step 6: Include state symbols if required:
- (s) = solid
- (l) = liquid
- (g) = gas
- (aq) = aqueous (dissolved in water)
Example with States:
\( \mathrm{2H_2(g) + O_2(g) \rightarrow 2H_2O(l)} \)
Common Symbols Used in Chemical Equations
| Symbol | Meaning | Example Usage |
|---|---|---|
| → | “Yields” or “produces” | \( \mathrm{Zn + 2HCl \rightarrow ZnCl_2 + H_2} \) |
| + | Separates reactants or products | \( \mathrm{Na + Cl_2 \rightarrow NaCl} \) |
| (s), (l), (g), (aq) | State of matter | \( \mathrm{H_2O(l)} \), \( \mathrm{CO_2(g)} \) |
| ↑ | Gas evolved | \( \mathrm{Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2↑} \) |
| ↓ | Precipitate formed | \( \mathrm{AgNO_3 + NaCl \rightarrow AgCl↓ + NaNO_3} \) |
Law of Conservation of Mass in Balancing
Statement: The mass of reactants = the mass of products because the number of atoms of each element is the same on both sides of the equation.
Example:
\( \mathrm{Fe + S \rightarrow FeS} \)
Balanced because: 1 atom of Fe and 1 atom of S appear on both sides.
Example:
Write the word and balanced chemical equation for the reaction between sodium and chlorine gas to form sodium chloride.
▶️ Answer / Explanation
Word Equation: Sodium + Chlorine → Sodium chloride
Chemical Equation: \( \mathrm{2Na + Cl_2 \rightarrow 2NaCl} \)
Step 1: Write formulas: \( \mathrm{Na} \), \( \mathrm{Cl_2} \), \( \mathrm{NaCl} \)
Step 2: Balance atoms — 2 Na and 2 Cl on both sides.
Final Answer: \( \mathrm{2Na + Cl_2 \rightarrow 2NaCl} \)
Example :
Hydrogen reacts with nitrogen to form ammonia gas. Write and balance the equation, including state symbols.
▶️ Answer / Explanation
Word Equation: Hydrogen + Nitrogen → Ammonia
Unbalanced: \( \mathrm{H_2(g) + N_2(g) \rightarrow NH_3(g)} \)
Step 1: Nitrogen: 2 on left, 1 on right → multiply \( \mathrm{NH_3} \) by 2.
Step 2: Hydrogen: now 6 on right, so multiply \( \mathrm{H_2} \) by 3.
Balanced Equation: \( \mathrm{N_2(g) + 3H_2(g) \rightarrow 2NH_3(g)} \)
Final Answer: Balanced and obeys conservation of mass.
Example:
Balance the following chemical equation and identify the type of reaction: \( \mathrm{FeCl_3 + NH_4OH \rightarrow Fe(OH)_3 + NH_4Cl} \)
▶️ Answer / Explanation
Step 1: Write number of atoms for each element on both sides:
Fe = 1, Cl = 3, N = 1, H = 5 (in NH₄OH), O = 1.
Step 2: Multiply \( \mathrm{NH_4OH} \) by 3 to balance Cl and H:
\( \mathrm{FeCl_3 + 3NH_4OH \rightarrow Fe(OH)_3 + 3NH_4Cl} \)
Step 3: Check balance: Fe = 1, Cl = 3, N = 3, H = 15, O = 3 → all balanced.
Type of Reaction: Double Displacement (precipitation)
Final Answer: \( \mathrm{FeCl_3 + 3NH_4OH \rightarrow Fe(OH)_3↓ + 3NH_4Cl} \)
