IB MYP 4-5 Chemistry -Writing and naming chemical formulas- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Writing and naming chemical formulas- Study Notes
Key Concepts
- Writing and Naming Chemical Formulas
Writing and Naming Chemical Formulas
Writing and Naming Chemical Formulas
A chemical formula shows the types and numbers of atoms present in a compound. It represents the simplest ratio in which elements combine chemically to form a substance.
Writing and naming chemical formulas correctly is essential for understanding reactions, predicting products, and communicating chemical information accurately.
Types of Compounds
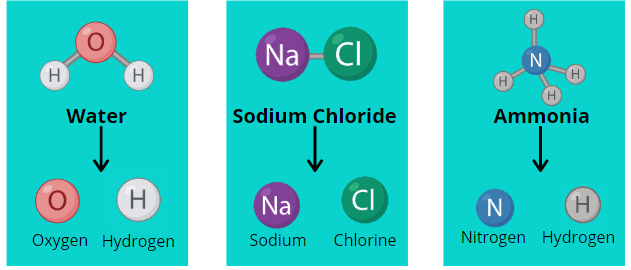
- Ionic Compounds: Formed by the transfer of electrons between metals and non-metals. (e.g., \( \mathrm{NaCl,\ MgO} \))
- Covalent Compounds: Formed by sharing of electrons between non-metals. (e.g., \( \mathrm{CO_2,\ H_2O} \))
Rules for Writing Formulas of Ionic Compounds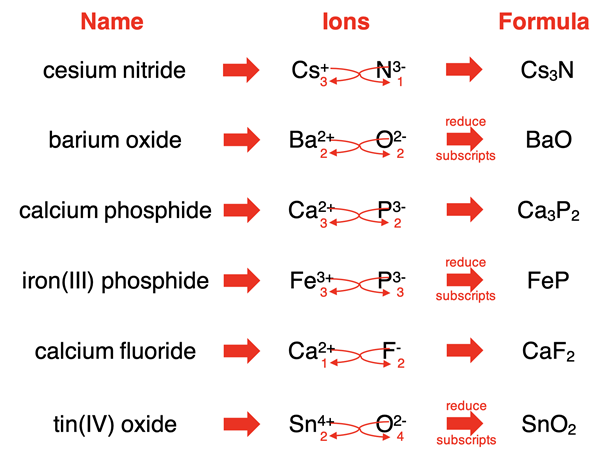
Step 1: Write the symbol of the metal (cation) first, then the non-metal (anion).
Step 2: Write the valency (charge) of each ion above its symbol.
Step 3: Balance the total positive and negative charges so that the compound is neutral overall.
Step 4: Write the formula by combining the symbols and using subscripts to show the ratio of ions.
Example:
- \( \mathrm{Na^+} \) and \( \mathrm{Cl^-} \) → one of each → \( \mathrm{NaCl} \)
- \( \mathrm{Mg^{2+}} \) and \( \mathrm{O^{2-}} \) → charges balance → \( \mathrm{MgO} \)
- \( \mathrm{Ca^{2+}} \) and \( \mathrm{Cl^-} \) → two Cl⁻ needed → \( \mathrm{CaCl_2} \)
Polyatomic ions: Some ions consist of more than one atom (act as a single charged unit). Examples include:
| Ion Name | Symbol | Charge |
|---|---|---|
| Ammonium | \( \mathrm{NH_4^+} \) | +1 |
| Hydroxide | \( \mathrm{OH^-} \) | −1 |
| Nitrate | \( \mathrm{NO_3^-} \) | −1 |
| Sulfate | \( \mathrm{SO_4^{2-}} \) | −2 |
| Carbonate | \( \mathrm{CO_3^{2-}} \) | −2 |
When combining polyatomic ions: If more than one polyatomic ion is required, use brackets around it. Example: \( \mathrm{Ca^{2+}} \) and \( \mathrm{NO_3^-} \) → \( \mathrm{Ca(NO_3)_2} \)
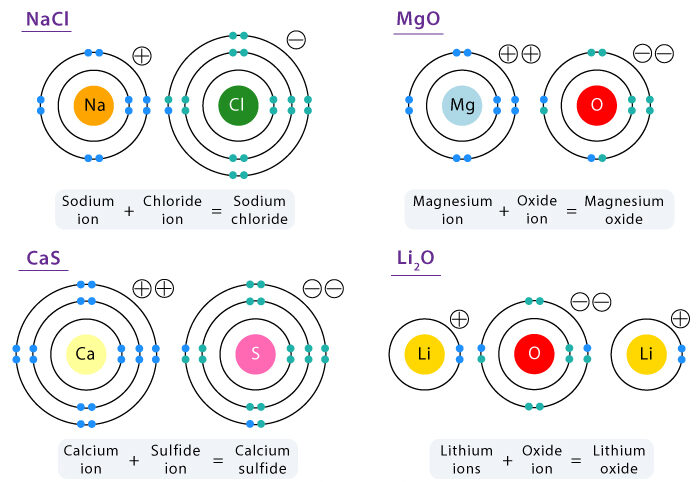
Naming Ionic Compounds
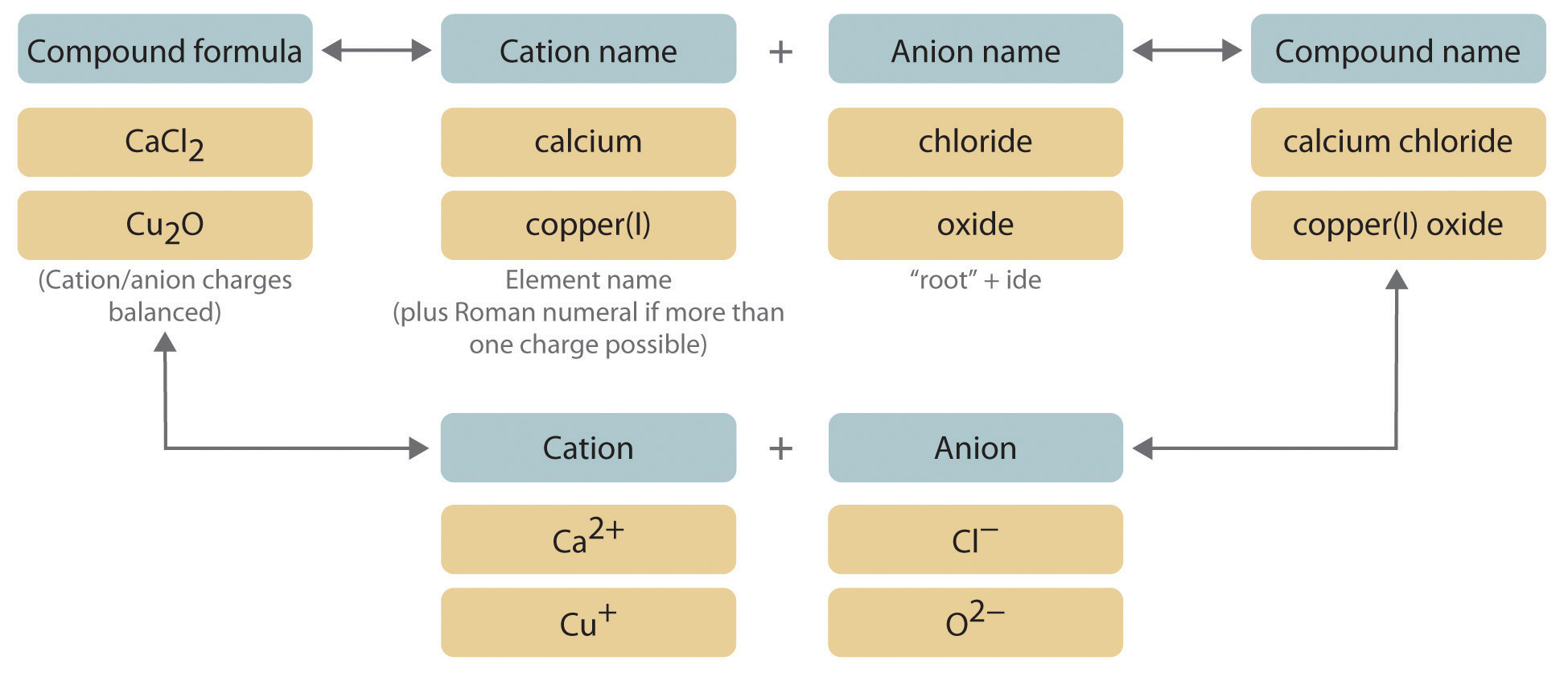
- Name the metal first, followed by the non-metal.
- Change the ending of the non-metal to “–ide.” e.g., \( \mathrm{NaCl} \): sodium chloride
- For compounds with polyatomic ions, use their full names: e.g., \( \mathrm{Na_2SO_4} \): sodium sulfate
- For transition metals, indicate the oxidation state (charge) with Roman numerals: e.g., \( \mathrm{FeCl_2} \): iron(II) chloride, \( \mathrm{FeCl_3} \): iron(III) chloride
Rules for Writing Formulas of Covalent Compounds
Step 1: Write symbols of the elements involved.
Step 2: Use prefixes to show how many atoms of each element are present.
Prefixes:
| Prefix | Number of Atoms |
|---|---|
| Mono– | 1 (often omitted for the first element) |
| Di– | 2 |
| Tri– | 3 |
| Tetra– | 4 |
| Penta– | 5 |
Examples:
- \( \mathrm{CO} \): carbon monoxide
- \( \mathrm{CO_2} \): carbon dioxide
- \( \mathrm{N_2O_3} \): dinitrogen trioxide
- \( \mathrm{PCl_5} \): phosphorus pentachloride
Common Mistakes to Avoid
- Do not write charges in the final formula (e.g., \( \mathrm{NaCl} \), not \( \mathrm{Na^+Cl^-} \)).
- For covalent compounds, use prefixes only — not charges.
- Always ensure ionic compounds are neutral overall.
Example :
Write the formula for calcium nitrate.
▶️ Answer / Explanation
Step 1: Calcium ion = \( \mathrm{Ca^{2+}} \), Nitrate ion = \( \mathrm{NO_3^-} \).
Step 2: Two nitrate ions are needed to balance +2 charge.
Step 3: Formula = \( \mathrm{Ca(NO_3)_2} \).
Final Answer: \( \mathrm{Ca(NO_3)_2} \)
Example :
Write the name and formula of a compound formed between aluminum and sulfur.
▶️ Answer / Explanation
Step 1: Aluminum forms \( \mathrm{Al^{3+}} \); sulfur forms \( \mathrm{S^{2-}} \).
Step 2: Cross charges → 2 aluminum and 3 sulfur atoms.
Step 3: Formula = \( \mathrm{Al_2S_3} \).
Final Answer: Aluminum sulfide (\( \mathrm{Al_2S_3} \)).
Example:
The compound ammonium sulfate is used in fertilizers. Write its formula and explain how it is derived.
▶️ Answer / Explanation
Step 1: Ammonium ion = \( \mathrm{NH_4^+} \); Sulfate ion = \( \mathrm{SO_4^{2-}} \).
Step 2: Two ammonium ions are needed to balance one sulfate ion.
Step 3: Formula = \( \mathrm{(NH_4)_2SO_4} \).
Final Answer: \( \mathrm{(NH_4)_2SO_4} \) — ammonium sulfate.
