IB MYP 4-5 Physics- Atmospheric pressure - Study Notes - New Syllabus
IB MYP 4-5 Physics-Atmospheric pressure – Study Notes
Key Concepts
- Atmospheric pressure
Atmospheric Pressure
Atmospheric Pressure
Atmospheric pressure is the force per unit area exerted by the weight of the air above a given point, caused by the Earth’s gravitational pull on the air molecules.
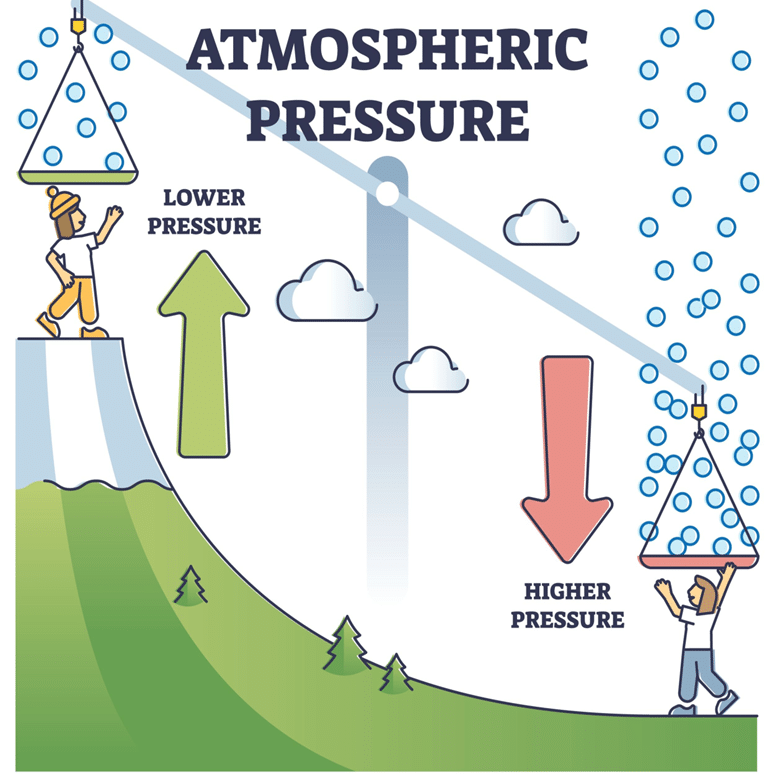
- The Earth’s atmosphere is composed of gases that have mass and therefore exert weight.
- Air exerts pressure in all directions—upward, downward, and sideways—because gas molecules move randomly and collide with surfaces.
- At sea level, the standard atmospheric pressure is approximately \( 1.013 \times 10^5 \, \text{Pa} \) (101.3 kPa), which is also equivalent to \( 760 \, \text{mmHg} \) or \( 1 \, \text{atm} \).
- Atmospheric pressure decreases with altitude because there is less air above a given point, meaning less weight pressing down.
- Pressure is measured using devices such as barometers and manometers.
Mathematical Relation:
\( P = \rho g h \)
Where: \( P \) = pressure due to air column (Pa) \( \rho \) = density of air (kg/m³) \( g \) = gravitational field strength (m/s²) \( h \) = height of air column (m)
Measurement:
Mercury Barometer:
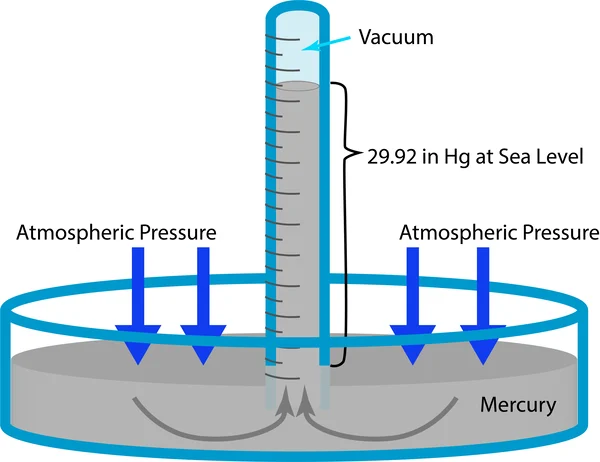
Uses a column of mercury; atmospheric pressure is indicated by the height of the mercury column.
Aneroid Barometer:

Uses a sealed metal chamber that expands/contracts with changes in pressure; movement is transferred to a dial.
Effects of Atmospheric Pressure:
- Boiling point of liquids changes with altitude—lower pressure means lower boiling point.
- Breathing at high altitudes becomes harder due to reduced oxygen partial pressure.
- It can crush objects if internal pressure is less than external atmospheric pressure (e.g., a sealed can cooled after heating).
Applications:
- Weather prediction using barometric pressure trends.
- Vacuum packaging and sealing.
- Aircraft cabin pressurization for passenger comfort at high altitudes.
Example:
The atmospheric pressure at a certain altitude is \( 8.5 \times 10^4 \, \text{Pa} \). If the density of air is \( 1.1 \, \text{kg/m}^3 \) and \( g = 9.8 \, \text{m/s}^2 \), estimate the height of the air column above that point.
▶️ Answer/Explanation
We use \( P = \rho g h \).
\( h = \dfrac{P}{\rho g} = \dfrac{8.5 \times 10^4}{1.1 \times 9.8} \)
\( h \approx 7870 \, \text{m} \)
\(\boxed{h \approx 7.87 \, \text{km}}\)
Example:
A barometer reading changes from \( 760 \, \text{mmHg} \) to \( 740 \, \text{mmHg} \). What is the drop in atmospheric pressure in Pascals? (Density of mercury \( = 13600 \, \text{kg/m}^3 \), \( g = 9.8 \, \text{m/s}^2 \)).
▶️ Answer/Explanation
Change in height: \( \Delta h = 20 \, \text{mm} = 0.020 \, \text{m} \)
Pressure change: \( \Delta P = \rho g \Delta h \)
\( \Delta P = 13600 \times 9.8 \times 0.020 \)
\( \Delta P \approx 2665.6 \, \text{Pa} \)
\(\boxed{\Delta P \approx 2.67 \, \text{kPa}}\)
