IB MYP 4-5 Physics- Atoms and subatomic particles- Study Notes - New Syllabus
IB MYP 4-5 Physics-Atoms and subatomic particles- Study Notes
Key Concepts
- Atoms and subatomic particles
Atoms and subatomic particles
Atoms and subatomic particles
Atoms are the basic building blocks of matter. They are extremely small and made up of subatomic particles: protons, neutrons, and electrons. The arrangement of these particles determines the atom’s structure and properties.
The Nucleus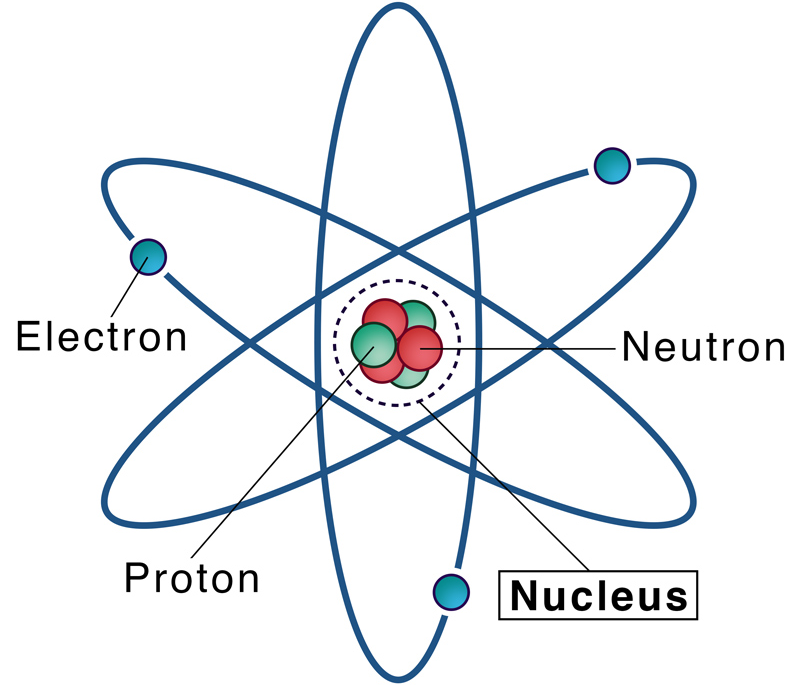
- The dense central part of the atom.
- Contains protons (positively charged) and neutrons (neutral).
- Nearly all the mass of the atom is concentrated in the nucleus.
- Radius of nucleus ≈ \(10^{-15}\,\text{m}\), very small compared to the size of the atom (\(10^{-10}\,\text{m}\)).
Protons (p⁺)
- Positively charged particles (\(+1\) charge).
- Charge: \(+1.6 \times 10^{-19}\, C\).
- Mass ≈ \(1.67 \times 10^{-27}\,\text{kg}\) (relative mass = 1).
- The number of protons = atomic number (Z), which defines the element.
- Found in the nucleus.
Neutrons (n⁰)
- Neutral particles (no charge).
- Charge = 0.
- Mass ≈ \(1.67 \times 10^{-27}\,\text{kg}\) (relative mass = 1).
- Provide stability to the nucleus by preventing proton–proton repulsion.
- Number of protons + neutrons = mass number (A).
- Found in the nucleus.
Electrons (e⁻)
- Negatively charged particles (\(-1\) charge).
- Charge: \(-1.6 \times 10^{-19}\, C\).
- Mass ≈ \(9.11 \times 10^{-31}\,\text{kg}\) (relative mass ≈ \( \dfrac{1}{1836} \)).
- Orbit the nucleus in shells/energy levels.
- Number of electrons = number of protons (in a neutral atom).
- Electrons determine chemical behavior and bonding.
Atomic Structure
- Atoms consist of a nucleus surrounded by electrons.
- Electrons are arranged in shells/energy levels (Bohr model).
- First shell holds up to 2 electrons, second up to 8, third up to 18, etc.
- Outermost electrons are called valence electrons, important for bonding.
- Atomic number (Z): Number of protons.
- Mass number (A): Number of protons + neutrons.
\( A = Z + N \), where \( N \) = number of neutrons.
- Neutral atom: Number of protons = number of electrons.
- Ions: Atoms that gain or lose electrons become charged (positive cations or negative anions).
Isotopes
Atoms of the same element (same number of protons, same \( Z \)) but different numbers of neutrons.
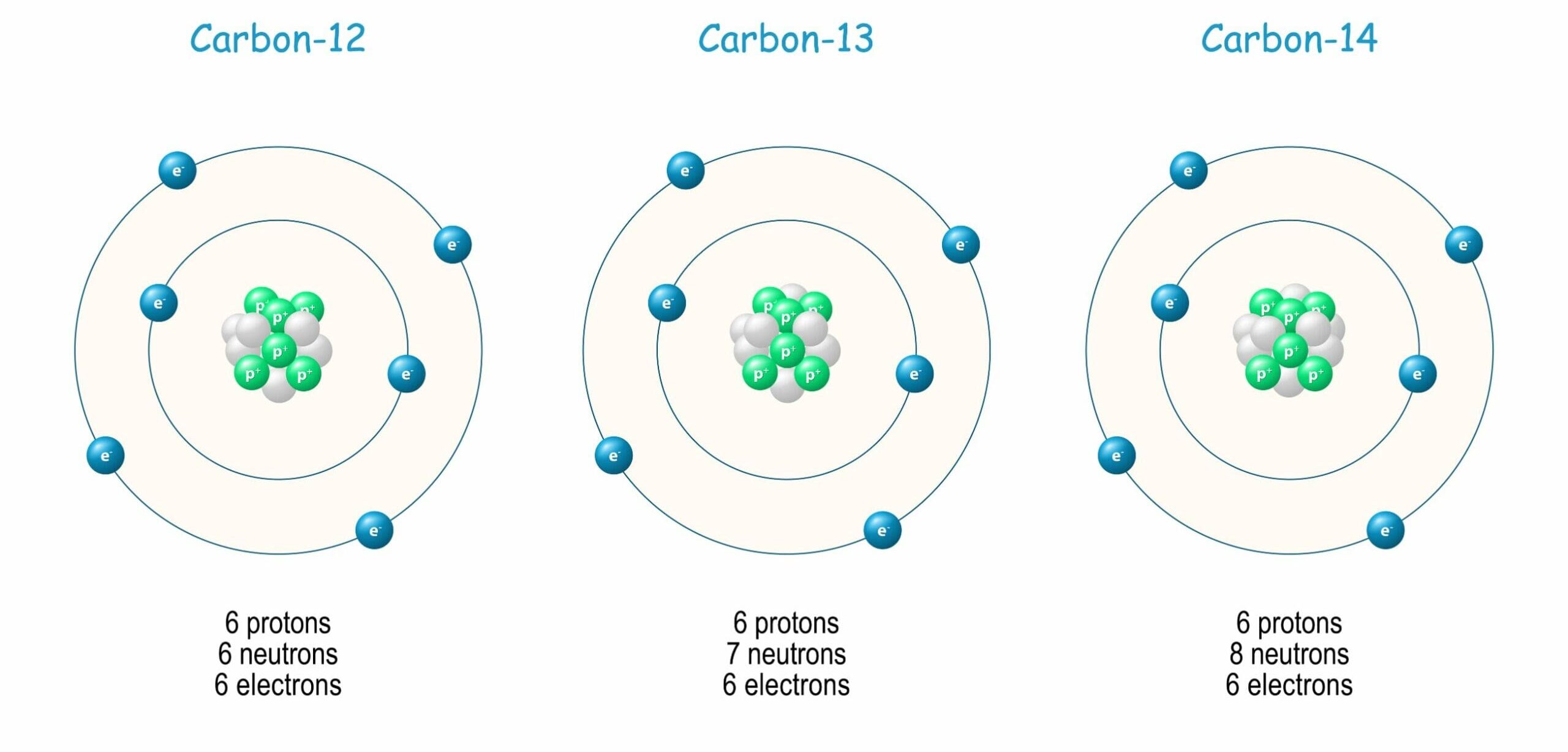
- Example: Carbon-12 and Carbon-14.
- Isotopes have identical chemical properties but different physical properties (mass, density, stability).
Ions
Formed when atoms gain or lose electrons.
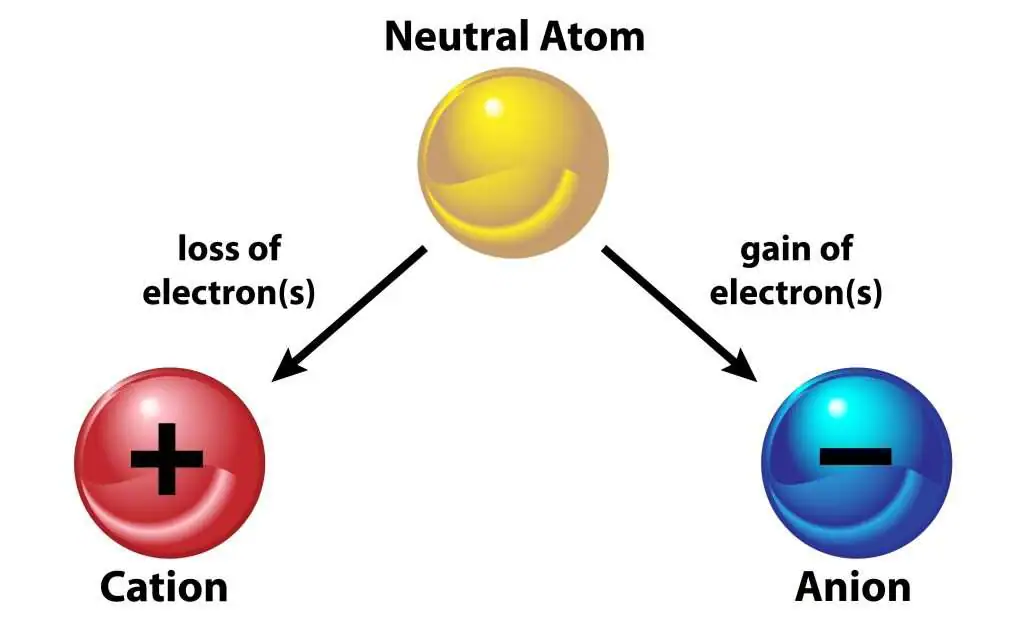
- Gain of electrons → negative ion (anion).
- Loss of electrons → positive ion (cation).
Subatomic Particles
| Particle | Symbol | Charge | Relative Mass | Exact Mass (kg) | Location |
|---|---|---|---|---|---|
| Proton | p⁺ | +1 | 1 | \(1.67 \times 10^{-27}\) | Nucleus |
| Neutron | n⁰ | 0 | 1 | \(1.67 \times 10^{-27}\) | Nucleus |
| Electron | e⁻ | -1 | 1/1836 | \(9.11 \times 10^{-31}\) | Electron shells/orbitals |
Example:
An atom has an atomic number of 11 and a mass number of 23. Determine the number of protons, neutrons, and electrons in this atom.
▶️ Answer/Explanation
Step 1: Atomic number (Z) = number of protons = 11.
Step 2: Mass number (A) = protons + neutrons = 23 → Neutrons = 23 – 11 = 12.
Step 3: For a neutral atom, number of electrons = number of protons = 11.
\(\boxed{\text{Protons = 11, Neutrons = 12, Electrons = 11}}\)
Example:
Carbon has two isotopes: Carbon-12 (\(^{12}C\)) and Carbon-14 (\(^{14}C\)). Compare the number of protons, neutrons, and electrons in both isotopes.
▶️ Answer/Explanation
Step 1: Atomic number of Carbon = 6 → Protons = 6.
Step 2: Electrons (neutral atom) = 6.
Step 3: Neutrons = Mass number – Protons.
For \(^{12}C\): Neutrons = \(12 – 6 = 6\).
For \(^{14}C\): Neutrons = \(14 – 6 = 8\).
Comparison:
\(\boxed{^{12}C: 6p, 6n, 6e \quad \text{vs.} \quad ^{14}C: 6p, 8n, 6e}\)
Example:
A sodium atom loses one electron to form an ion. Write down the symbol of this ion and determine its number of protons, neutrons, and electrons (given: mass number = 23, atomic number = 11).
▶️ Answer/Explanation
Step 1: Atomic number = 11 → Protons = 11.
Step 2: Mass number = 23 → Neutrons = \(23 – 11 = 12\).
Step 3: Neutral sodium atom has 11 electrons. Losing one electron → 10 electrons.
Step 4: Ion formed = \(Na^{+}\).
\(\boxed{Na^{+}: 11p, 12n, 10e}\)
Example:
Chlorine has isotopes Cl-35 and Cl-37. Explain their similarities and differences.
▶️ Answer/Explanation
Step 1: Both isotopes have 17 protons (same element).
Step 2: Cl-35 has 18 neutrons, Cl-37 has 20 neutrons.
Step 3: Same chemical properties (same number of electrons).
Step 4: Different physical properties like mass and density.
Final Answer: Isotopes of chlorine differ in neutron number but have identical chemistry.
Example:
An oxygen ion \( O^{2-} \) has 8 protons, 8 neutrons, and 10 electrons. Why is it negatively charged?
▶️ Answer/Explanation
Step 1: Neutral oxygen atom → 8 protons = 8 electrons.
Step 2: Ion has 10 electrons (2 extra).
Step 3: Extra electrons → overall charge = \(-2\).
Final Answer: The oxygen ion is \(\boxed{2^-}\) because it gained two extra electrons.
