IB MYP 4-5 Physics- Electric Force- Study Notes - New Syllabus
IB MYP 4-5 Physics-Electric Force- Study Notes
Key Concepts
- Electric Force
Electric Force
Electric Force
The electric force is the force of attraction or repulsion between two charged objects. It is a type of non-contact force and one of the fundamental interactions in nature.
- Like charges (positive–positive or negative–negative) repel each other.
- Unlike charges (positive–negative) attract each other.
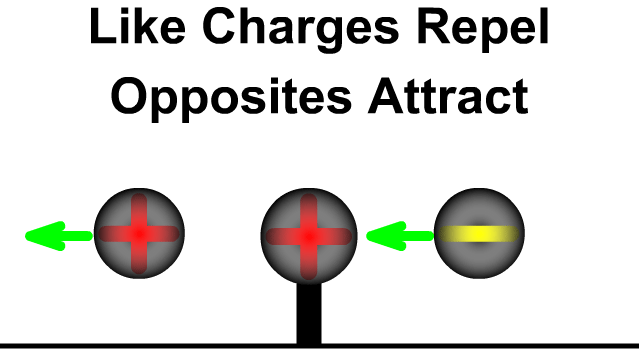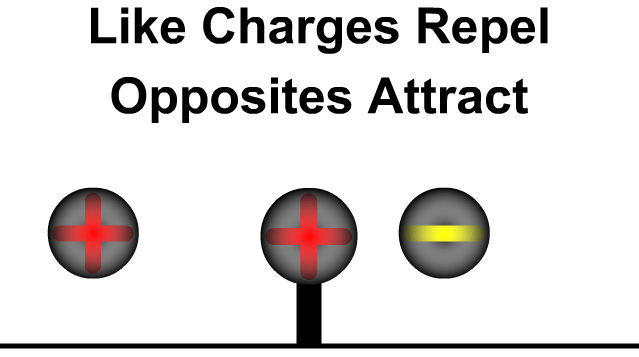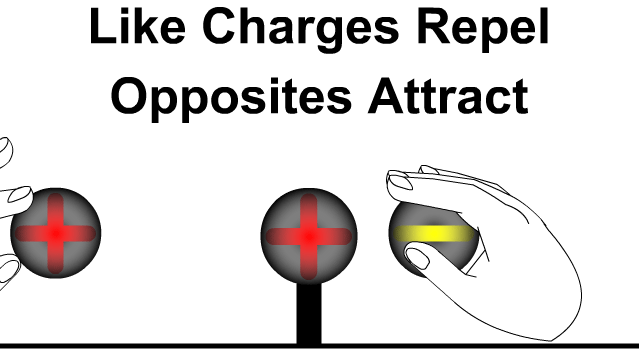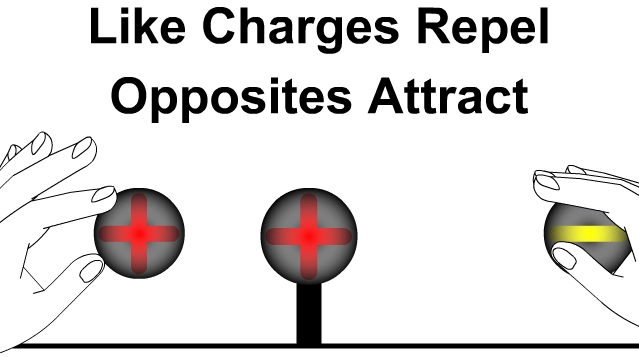
- The force acts along the line joining the two charges.

The electric force between two point charges is given by Coulomb’s Law:
\( F = k \dfrac{q_1 q_2}{r^2} \)
where:
- \( F \) = electric force (N)
- \( q_1, q_2 \) = magnitudes of the charges (C)
- \( r \) = distance between charges (m)
- \( k = 9 \times 10^9 \, \text{N·m}^2/\text{C}^2 \) (Coulomb’s constant)
Key Notes
- The electric force is stronger when charges are larger and/or closer together.
- It decreases rapidly with distance (inversely proportional to \( r^2 \)).
- Electric forces are much stronger than gravitational forces for small particles like electrons and protons.
Example:
Two charges, \( q_1 = +2.0 \, \mu C \) and \( q_2 = -3.0 \, \mu C \), are placed 0.5 m apart. Calculate the electric force between them.
▶️ Answer/Explanation
Using Coulomb’s Law: \( F = k \dfrac{q_1 q_2}{r^2} \)
\( F = (9 \times 10^9) \dfrac{(2.0 \times 10^{-6})(3.0 \times 10^{-6})}{(0.5)^2} \)
\( F = (9 \times 10^9)(1.2 \times 10^{-11}) / 0.25 \)
\( F = 0.432 \, \text{N} \).
Since the charges are opposite, the force is attractive. Final Answer: \( \boxed{0.432 \, \text{N (attractive)}} \).
Example:
Two electrons are separated by \( 1.0 \times 10^{-10} \, \text{m} \). Calculate the electric force between them (electron charge \( e = 1.6 \times 10^{-19} \, C \)).
▶️ Answer/Explanation
Using Coulomb’s Law: \( F = k \dfrac{e^2}{r^2} \)
\( F = (9 \times 10^9) \dfrac{(1.6 \times 10^{-19})^2}{(1.0 \times 10^{-10})^2} \)
\( F = (9 \times 10^9)(2.56 \times 10^{-38}) / (1.0 \times 10^{-20}) \) \( F = 2.3 \times 10^{-8} \, \text{N} \). Since both charges are negative, the force is repulsive.
Final Answer: \( \boxed{2.3 \times 10^{-8} \, \text{N (repulsive)}} \).
Example:
Compare the electric force and gravitational force between a proton and an electron separated by \( 5.0 \times 10^{-11} \, \text{m} \). (Data: \( e = 1.6 \times 10^{-19} \, C \), \( m_p = 1.67 \times 10^{-27} \, kg \), \( m_e = 9.11 \times 10^{-31} \, kg \), \( G = 6.67 \times 10^{-11} \)).
▶️ Answer/Explanation
Electric force: \( F_e = k \dfrac{e^2}{r^2} \)
\( = (9 \times 10^9) \dfrac{(1.6 \times 10^{-19})^2}{(5.0 \times 10^{-11})^2} \) \( = 9.2 \times 10^{-8} \, \text{N} \).
Gravitational force: \( F_g = G \dfrac{m_p m_e}{r^2} \)
\( = (6.67 \times 10^{-11}) \dfrac{(1.67 \times 10^{-27})(9.11 \times 10^{-31})}{(5.0 \times 10^{-11})^2} \) \( = 3.6 \times 10^{-47} \, \text{N} \).
Comparison: \( F_e / F_g \approx 2.6 \times 10^{39} \). The electric force is astronomically stronger than the gravitational force at atomic scales.
