IB MYP 4-5 Physics- Electromagnetic spectrum- Study Notes - New Syllabus
IB MYP 4-5 Physics-Electromagnetic spectrum- Study Notes
Key Concepts
- Electromagnetic spectrum
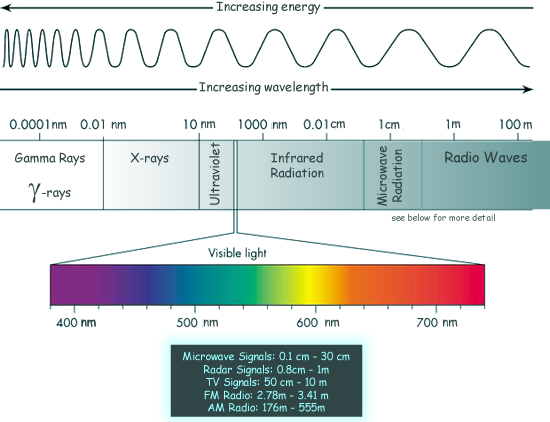
Electromagnetic Spectrum
Electromagnetic Spectrum
The electromagnetic (EM) spectrum is the complete range of electromagnetic waves arranged according to their wavelength (\( \lambda \)) or frequency (\( f \)). All EM waves travel at the same speed in a vacuum:
\( c = 3.0 \times 10^{8} \, \text{m/s} \)
General Properties of EM Waves:
- They are transverse waves (oscillating electric and magnetic fields at right angles).
- They can travel through vacuum (do not need a medium).
- They transfer energy from one place to another.
- All have the same speed \(c\) in vacuum but different frequencies and wavelengths.
- Relationship between speed, frequency, and wavelength:
- \( c = f \lambda \)
Order of the Electromagnetic Spectrum:
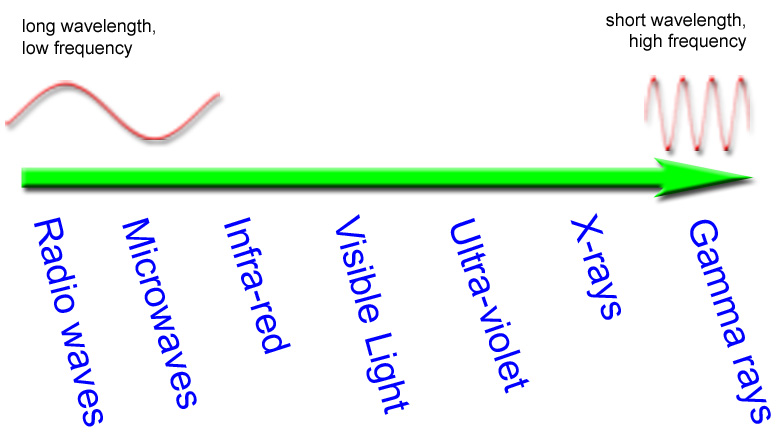
How do I remember all this?
Try: Rabbits | meaning: Radio |
|---|
Wavelength and Frequency Ranges:
| Type | Approx. Wavelength Range | Approx. Frequency Range | Common Uses |
|---|---|---|---|
| Radio Waves | \( > 1 \, \text{m} \) | \( < 3 \times 10^{8} \, \text{Hz} \) | Broadcasting, communication |
| Microwaves | \( 1 \, \text{mm} – 1 \, \text{m} \) | \( 3 \times 10^{8} – 3 \times 10^{11} \, \text{Hz} \) | Cooking, radar, satellites |
| Infrared | \( 700 \, \text{nm} – 1 \, \text{mm} \) | \( 3 \times 10^{11} – 4 \times 10^{14} \, \text{Hz} \) | Remote controls, thermal imaging |
| Visible Light | \( 400 – 700 \, \text{nm} \) | \( 4 \times 10^{14} – 7.5 \times 10^{14} \, \text{Hz} \) | Vision, photography |
| Ultraviolet | \( 10 \, \text{nm} – 400 \, \text{nm} \) | \( 7.5 \times 10^{14} – 3 \times 10^{16} \, \text{Hz} \) | Sterilisation, sun tanning |
| X-Rays | \( 0.01 – 10 \, \text{nm} \) | \( 3 \times 10^{16} – 3 \times 10^{19} \, \text{Hz} \) | Medical imaging, security |
| Gamma Rays | \( < 0.01 \, \text{nm} \) | \( > 3 \times 10^{19} \, \text{Hz} \) | Cancer treatment, sterilisation |
Key Safety Note:
High-frequency, short-wavelength EM waves (UV, X-rays, gamma rays) carry enough energy to be harmful to living cells, causing burns, cell damage, or cancer. Proper protection is essential.
Example:
A radio station broadcasts at a frequency of \( 100 \, \text{MHz} \). Calculate the wavelength of the radio waves in air. (Take \( c = 3.0 \times 10^{8} \, \text{m/s} \)).
▶️ Answer/Explanation
Step 1: Recall the wave equation:
\( c = f \lambda \)
Step 2: Rearrange for wavelength:
\( \lambda = \dfrac{c}{f} \)
Step 3: Substitute values:
\( \lambda = \dfrac{3.0 \times 10^{8}}{100 \times 10^{6}} \)
\( \lambda = 3.0 \, \text{m} \)
Final Answer:
\(\boxed{3.0 \, \text{m}}\)
Example:
A hospital uses X-rays with a wavelength of \( 0.1 \, \text{nm} \). Calculate their frequency. (Take \( c = 3.0 \times 10^{8} \, \text{m/s} \)).
▶️ Answer/Explanation
Step 1: Use the wave equation:
\( f = \dfrac{c}{\lambda} \)
Step 2: Convert wavelength into meters:
\( 0.1 \, \text{nm} = 0.1 \times 10^{-9} = 1.0 \times 10^{-10} \, \text{m} \)
Step 3: Substitute values:
\( f = \dfrac{3.0 \times 10^{8}}{1.0 \times 10^{-10}} \)
\( f = 3.0 \times 10^{18} \, \text{Hz} \)
Final Answer:
\(\boxed{3.0 \times 10^{18} \, \text{Hz}}\)
Example:
Why are gamma rays more dangerous to living cells compared to radio waves?
▶️ Answer/Explanation
Step 1: Gamma rays have very short wavelengths and very high frequencies.
Step 2: The energy of an EM wave photon is given by:
\( E = hf \) (where \( h \) is Planck’s constant)
Step 3: Since gamma rays have much higher frequency than radio waves, each gamma photon carries much more energy.
Step 4: High-energy photons can ionise atoms in cells, damaging DNA and potentially causing cancer, while radio waves are non-ionising and carry much less energy.
Final Answer:
\(\boxed{\text{Gamma rays are dangerous because their photons are highly energetic and can damage living tissue, unlike radio waves.}}\)
