IB MYP 4-5 Physics- Expansion of solids, liquids, and gases- Study Notes - New Syllabus
IB MYP 4-5 Physics-Expansion of solids, liquids, and gases- Study Notes
Key Concepts
- Expansion of solids, liquids, and gases
Expansion of Solids, Liquids, and Gases
Introduction to Expansion
When a material is heated, the average kinetic energy of its particles increases. This causes them to vibrate or move more vigorously, leading to an increase in the average separation between particles. As a result, most substances expand when heated and contract when cooled.

This phenomenon is known as thermal expansion.
Types of Expansion
- Linear Expansion (Solids): Increase in length of a solid when heated.
- Area Expansion (Solids): Increase in surface area when a solid is heated.
- Volumetric Expansion (Solids, Liquids, Gases): Increase in volume when temperature rises.
Linear Expansion in Solids
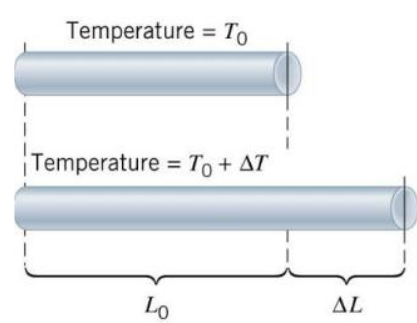
The change in length is given by:
\(\Delta L = \alpha L_0 \Delta T\)
- \(\Delta L\) = change in length
- \(\alpha\) = coefficient of linear expansion (per °C or per K)
- \(L_0\) = original length
- \(\Delta T\) = temperature change
Examples: Metal rods, rails, bridges expand in summer and contract in winter.
Area Expansion (Solids)
\(\Delta A = 2\alpha A_0 \Delta T\)
- \(\Delta A\) = change in area
- \(A_0\) = original area
Volumetric Expansion
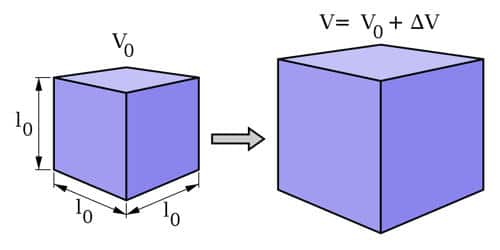
\(\Delta V = \beta V_0 \Delta T\)
- \(\beta\) = coefficient of volumetric expansion (for solids, \(\beta \approx 3\alpha\))
Expansion of Liquids
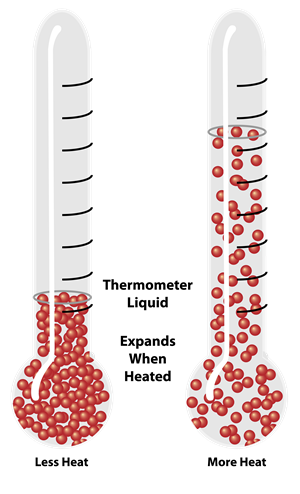
- Liquids have no fixed shape, so only volumetric expansion is considered.
- The expansion is affected by the expansion of the container, so we measure the apparent expansion (observed) and true expansion (actual).
\(\text{True Expansion} = \text{Apparent Expansion} + \text{Expansion of Container}\)
Expansion of Gases
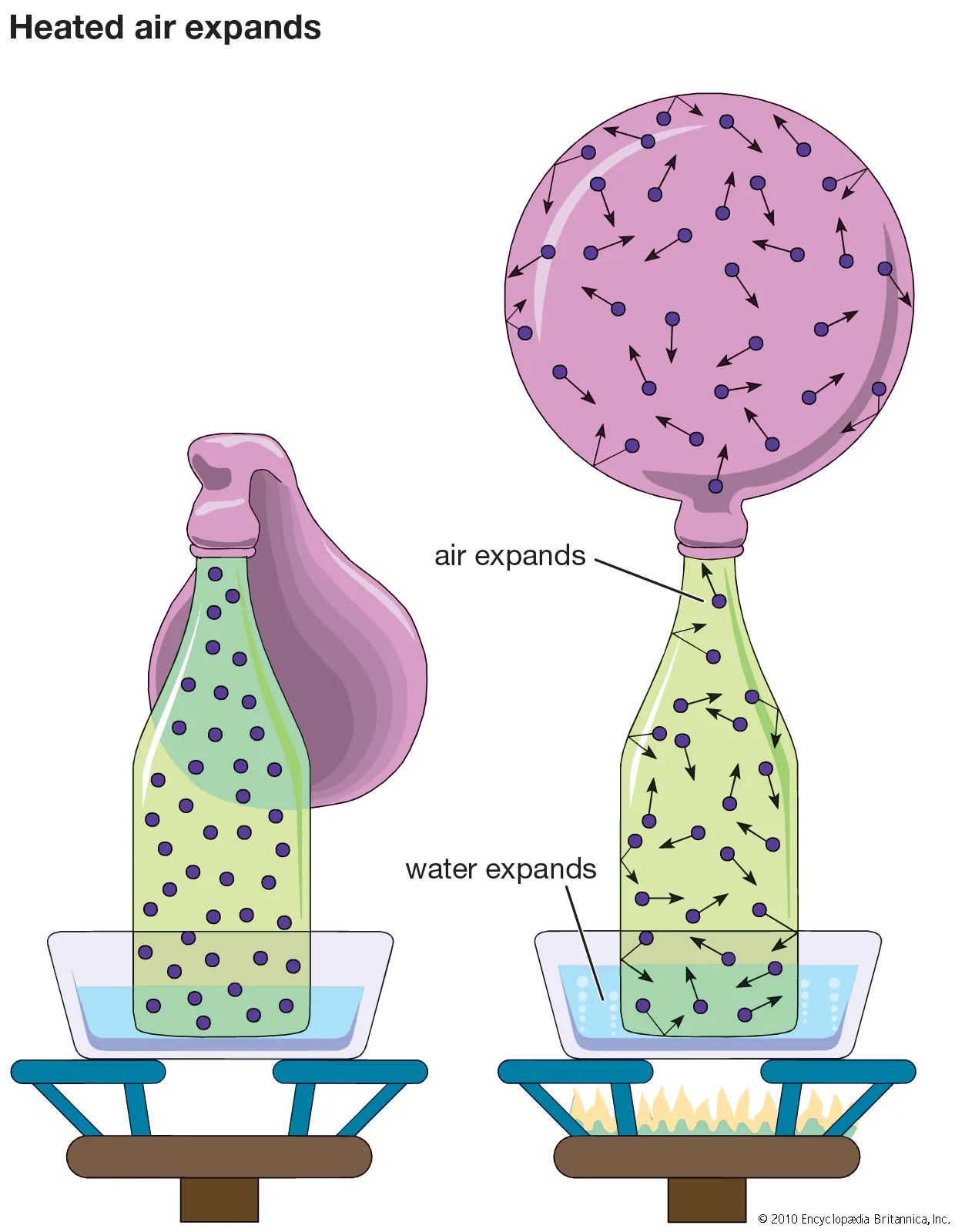
- Gases expand much more than solids or liquids for the same temperature change.
- Expansion of gases follows gas laws: Charles’s Law, Boyle’s Law, and the Pressure Law.
- Under constant pressure, volume is directly proportional to temperature (\(V \propto T\)).
Practical Applications of Thermal Expansion
- Gaps left between railway tracks to prevent buckling.
- Expansion joints in bridges and buildings.
- Bimetallic strips in thermostats.
- Mercury or alcohol thermometers.
| Material | Type of Expansion | Coefficient of Expansion | Typical Magnitude |
|---|---|---|---|
| Solid (Steel) | Linear & Volumetric | \(\alpha \approx 1.2 \times 10^{-5} \ \text{K}^{-1}\) | Small |
| Liquid (Water) | Volumetric | \(\beta \approx 2.1 \times 10^{-4} \ \text{K}^{-1}\) | Moderate |
| Gas (Air) | Volumetric | \(\beta \approx 3.4 \times 10^{-3} \ \text{K}^{-1}\) | Very Large |
Example:
A steel railway track of length \(L = 50 \ \text{m}\) expands when the temperature increases from \(10^\circ\text{C}\) to \(40^\circ\text{C}\). The coefficient of linear expansion of steel is \(\alpha = 1.2 \times 10^{-5} \ \text{K}^{-1}\). Calculate the increase in length.
▶️ Answer/Explanation
Step 1: Formula for linear expansion:
\(\Delta L = \alpha L \Delta T\)
Step 2: Substituting values:
\(\Delta L = (1.2 \times 10^{-5}) \times (50) \times (40 – 10)\)
\(\Delta L = (1.2 \times 10^{-5}) \times 50 \times 30\)
\(\Delta L = 1.8 \times 10^{-2} \ \text{m} = 1.8 \ \text{cm}\)
Final Answer: \(\boxed{1.8 \ \text{cm}}\) increase in length.
Example:
A container holds \(2.0 \ \text{L}\) of ethanol at \(20^\circ\text{C}\). The coefficient of volumetric expansion of ethanol is \(\beta = 1.1 \times 10^{-4} \ \text{K}^{-1}\). Find the new volume if the temperature rises to \(50^\circ\text{C}\).
▶️ Answer/Explanation
Step 1: Formula for volumetric expansion:
\(\Delta V = \beta V \Delta T\)
Step 2: Substituting values:
\(\Delta V = (1.1 \times 10^{-4}) \times (2.0) \times (50 – 20)\)
\(\Delta V = (1.1 \times 10^{-4}) \times 2.0 \times 30\)
\(\Delta V = 6.6 \times 10^{-3} \ \text{L}\)
Step 3: New volume:
\(V_{\text{new}} = 2.0 + 0.0066 = 2.0066 \ \text{L}\)
Final Answer: \(\boxed{2.0066 \ \text{L}}\)
Example:
A fixed amount of gas is kept at constant pressure in a cylinder. Its volume at \(27^\circ\text{C}\) is \(0.020 \ \text{m}^3\). If the temperature rises to \(127^\circ\text{C}\), calculate the final volume, assuming ideal gas behavior.
▶️ Answer/Explanation
Step 1: At constant pressure, gas volume follows Charles’ Law:
\(\dfrac{V_1}{T_1} = \dfrac{V_2}{T_2}\)
Step 2: Convert °C to Kelvin:
\(T_1 = 27 + 273 = 300 \ \text{K}\)
\(T_2 = 127 + 273 = 400 \ \text{K}\)
Step 3: Substituting values:
\(\dfrac{0.020}{300} = \dfrac{V_2}{400}\)
\(V_2 = \dfrac{0.020 \times 400}{300} = 0.0267 \ \text{m}^3\)
Final Answer: \(\boxed{0.0267 \ \text{m}^3}\)
