IB MYP 4-5 Physics- Half-life- Study Notes - New Syllabus
IB MYP 4-5 Physics-Half-life- Study Notes
Key Concepts
- Half-life
Half-Life
Half-Life (\(t_{1/2}\))
The half-life of a radioactive isotope is the time required for half of the nuclei in a sample to decay.
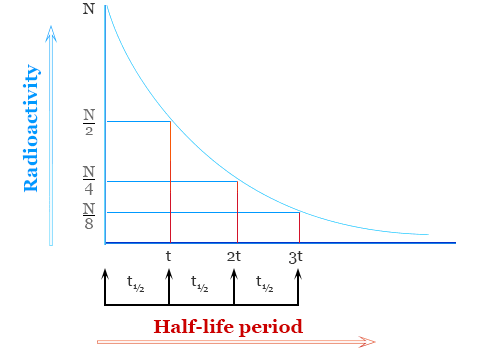
- It does not depend on:
- Amount of material.
- Physical conditions (temperature, pressure, etc.).
- The number of undecayed nuclei decreases exponentially with time.
$ N(t) = N_0 \, e^{-\lambda t} $
Where:
- \(N(t)\) = number of nuclei at time \(t\)
- \(N_0\) = initial number of nuclei
- \(\lambda\) = decay constant (probability of decay per unit time)
Relationship between half-life and decay constant: $ t_{1/2} = \dfrac{\ln 2}{\lambda} $
Points about Half-Life:
- After one half-life → \( \dfrac{1}{2} \) of the nuclei remain.
- After two half-lives → \( \dfrac{1}{4} \) remain.
- After three half-lives → \( \dfrac{1}{8} \) remain, and so on.
- This pattern is exponential decay, not linear.
Applications of Half-Life:
- Carbon dating: Using \(^{14}\text{C}\) to estimate the age of fossils and artifacts (useful up to ~50,000 years).
- Medical tracers: Using isotopes like \(^{99m}\text{Tc}\) (technetium-99m) with short half-lives for imaging, to minimize patient exposure.
- Nuclear waste management: Knowing the half-life helps determine safe storage times.
Example
A sample has mass \(N_0 = 80\,\text{g}\) of an isotope with half-life \(t_{1/2}=6\,\text{h}\). How much remains after \(t=24\,\text{h}\)?
▶️ Solution
Number of half-lives: \(n=\dfrac{t}{t_{1/2}}=\dfrac{24}{6}=4\).
\(N = N_0\left(\dfrac{1}{2}\right)^n = 80\left(\dfrac{1}{2}\right)^4 = 80 \times \dfrac{1}{16} = 5\,\text{g}\).
Answer: \(\boxed{5\,\text{g}}\).
Example
An activity drops from \(A_0=6400\,\text{Bq}\) to \(A=800\,\text{Bq}\) in \(t=9\,\text{h}\). Find \(t_{1/2}\).
▶️ Solution
Use \(A = A_0\left(\dfrac{1}{2}\right)^{t/t_{1/2}} \Rightarrow \dfrac{A}{A_0}=\left(\dfrac{1}{2}\right)^{t/t_{1/2}}\).
\(\dfrac{800}{6400}=\dfrac{1}{8}=\left(\dfrac{1}{2}\right)^3\Rightarrow \dfrac{t}{t_{1/2}}=3 \Rightarrow t_{1/2}=\dfrac{9\,\text{h}}{3}=3\,\text{h}\).
Answer: \(\boxed{t_{1/2}=3\,\text{h}}\).
Example
How long until only \(10\%\) of a sample remains, if \(t_{1/2}=5\,\text{days}\)?
▶️ Solution
\(\dfrac{N}{N_0}=0.10=\left(\dfrac{1}{2}\right)^{t/t_{1/2}}\).
Take logs: \(\ln(0.10)=\dfrac{t}{t_{1/2}}\ln\!\left(\dfrac{1}{2}\right)\Rightarrow t=t_{1/2}\,\dfrac{\ln(0.10)}{\ln(1/2)}\).
\(t=5\,\text{d}\times \dfrac{\ln 0.10}{\ln 0.5}\approx 5\times \dfrac{-2.3026}{-0.6931}\approx 5\times 3.322\approx 16.61\,\text{days}\).
Answer: \(\boxed{\approx 16.6\,\text{days}}\).
