IB MYP 4-5 Physics- Ionizing effect of radiation and its detection- Study Notes - New Syllabus
IB MYP 4-5 Physics-Ionizing effect of radiation and its detection- Study Notes
Key Concepts
- Ionizing effect of radiation and its detection
Ionisation and Radiation
Ionisation and Radiation
Ionisation is the process in which an atom or molecule gains or loses electrons, forming charged particles called ions.
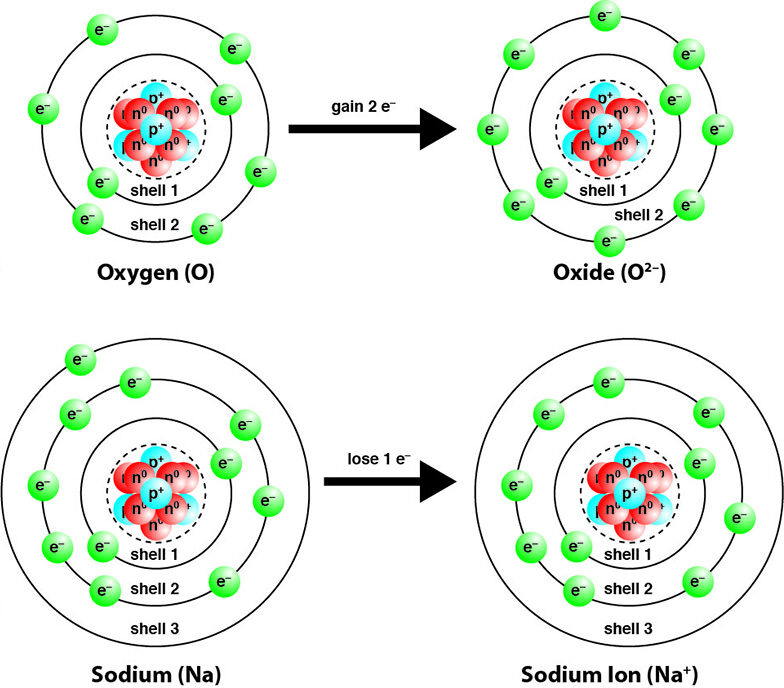
- Ionising radiation is radiation that has enough energy to remove tightly bound electrons from atoms.
- This process can damage living tissues by breaking chemical bonds in DNA, proteins, and other molecules.
Types of Ionising Radiation

Alpha (α) particles
- Strongly ionising (due to large mass and +2 charge).
- Can remove electrons from many atoms along a short path.
- Low penetration: stopped by paper or skin.
Beta (β) particles
- Moderately ionising (smaller mass and charge than alpha).
- Can penetrate further than alpha (stopped by thin aluminium).
Gamma (γ) rays
- Weakly ionising (no mass, no charge, only transfers energy).
- Very penetrating: requires thick lead or concrete to reduce intensity.
Mechanism of Ionisation
When ionising radiation collides with an atom:
- Electrons may be knocked out of the atom → atom becomes a positive ion.
- The free electron can also collide with other atoms, causing secondary ionisation.
Ionisation causes chemical and biological changes in matter.
Effects of Ionising Radiation
Biological effects:
- Damage to DNA → mutations → cancer risk.
- Cell death or malfunction.
- Radiation sickness at high doses.
Practical effects:
- Can be used to kill bacteria (sterilisation).
- Can damage electronic devices through ionisation.
Detection of Ionising Radiation
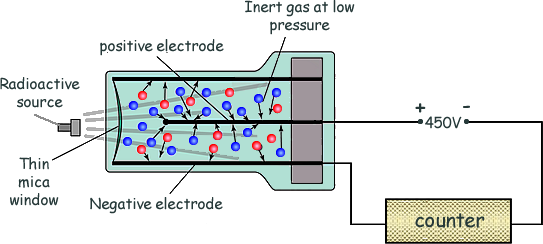
- Geiger–Müller (GM) tube: detects ionisation in a gas, producing a click for each particle.
- Cloud chamber: shows ionising tracks of radiation in supersaturated vapour.
- Photographic film: darkens when exposed to ionising radiation (used in film badges).
Example
Rank \(\alpha, \beta, \gamma\) by (a) ionising power and (b) penetration. Justify briefly.
▶️ Solution
(a) Ionising power: \(\alpha \gt \beta \gt \gamma\) (large charge \(+2\) and mass → dense energy loss along short path).
(b) Penetration: \(\gamma \gt \beta \gt \alpha\) (photons have no charge/mass, so fewer interactions per unit length).
Example
Pick the best primary shield and key precaution for each source in a school lab: \(\alpha\), \(\beta\), \(\gamma\).
▶️ Solution
\(\alpha\): paper/plastic container; precaution—prevent ingestion/inhalation (use sealed source, tongs).
\(\beta\): few mm Al or acrylic; precaution—use gloves/tongs; avoid high-Z shields to limit bremsstrahlung X-rays.
\(\gamma\): thick lead/concrete; precaution—time–distance–shielding, use dosimeter/remote handling.
Example
A GM tube reads high counts through paper, moderate through 3 mm Al, and still detects counts through 2 cm lead. Which radiations are present?
▶️ Solution
High counts through paper → not pure \(\alpha\) (paper would stop it). Moderate reduction with Al → indicates \(\beta\) being absorbed.
Counts persisting after 2 cm lead → presence of \(\gamma\) (high penetration).
Conclusion: Mixture of \(\beta\) and \(\gamma\) radiation.
