IB MYP 4-5 Physics- Nuclear energy- Study Notes - New Syllabus
IB MYP 4-5 Physics-Nuclear energy- Study Notes
Key Concepts
- Nuclear energy
Nuclear Energy
Concept of Nuclear Energy
Nuclear energy is the energy released from the nucleus of an atom during nuclear reactions.
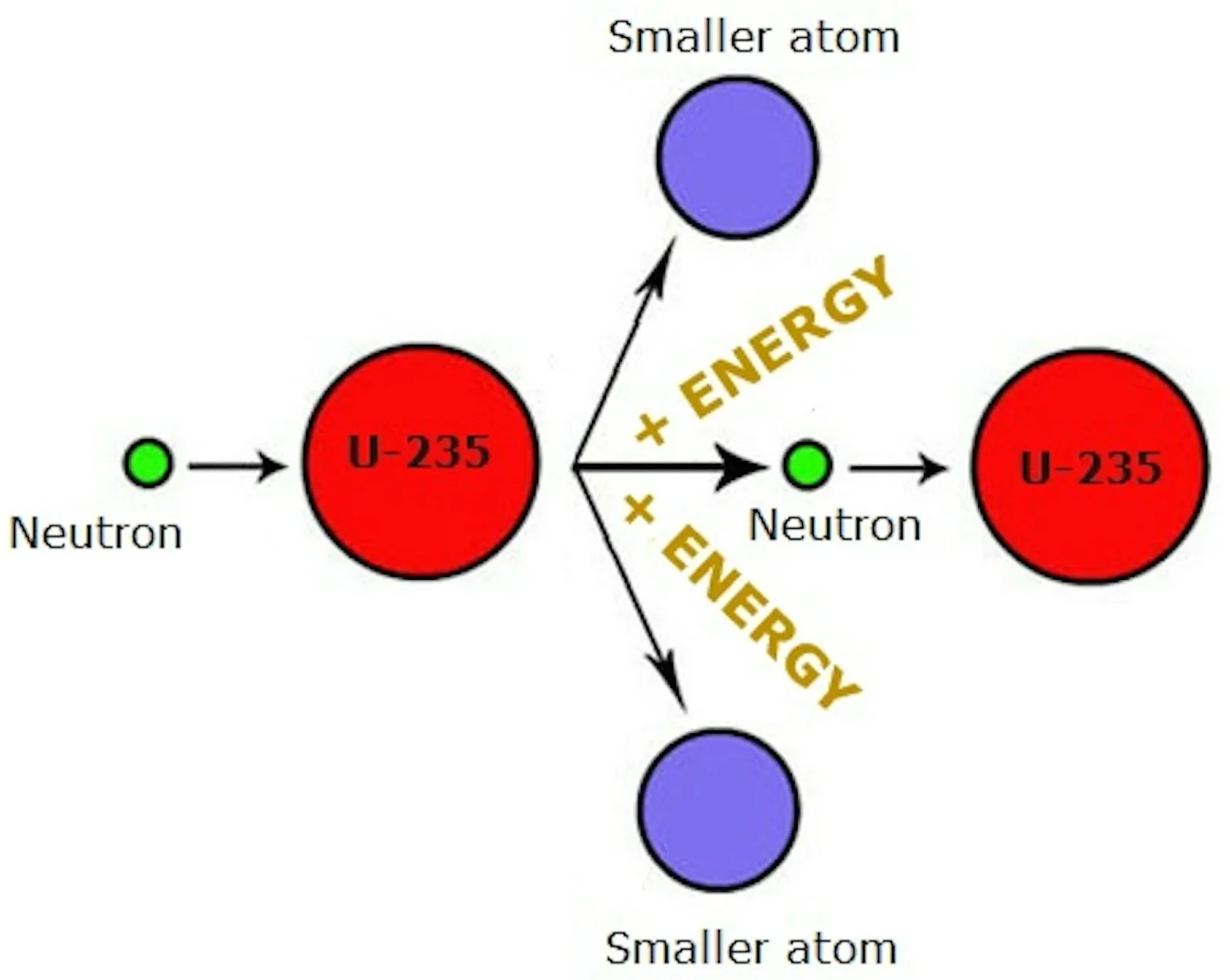
- It comes from two main processes:
- Nuclear Fission: Splitting a heavy nucleus (e.g., uranium-235 or plutonium-239) into smaller nuclei, releasing a large amount of energy.
- Nuclear Fusion: Combining light nuclei (e.g., hydrogen isotopes) to form a heavier nucleus, releasing even greater amounts of energy.
Nuclear Fission
Occurs when a heavy nucleus absorbs a neutron and becomes unstable.
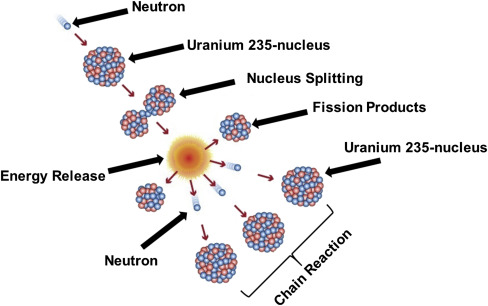
- The nucleus splits into two smaller nuclei, releasing energy, more neutrons, and radiation.
- The released neutrons can cause further fission → chain reaction.
Example reaction: \( \, ^{235}\text{U} + ^{1}\text{n} \;\;\rightarrow \;\; ^{92}\text{Kr} + ^{141}\text{Ba} + 3 \, ^{1}\text{n} + \text{Energy} \)
Applications: Nuclear power plants, atomic bombs.
Advantages: Produces large amounts of energy; no greenhouse gases during operation.
Disadvantages: Produces long-lived radioactive waste; risk of accidents; potential misuse for weapons.
Example:
A uranium-235 nucleus absorbs a neutron and undergoes fission. Explain why this reaction can lead to a chain reaction, and why control rods are necessary in a nuclear reactor.
▶️ Answer/Explanation
Each fission releases additional neutrons, which can strike other uranium-235 nuclei, causing more fission events. This creates a chain reaction.
Without control, the reaction rate grows exponentially, leading to overheating or even an explosion.
Control rods (made of boron or cadmium) absorb excess neutrons, ensuring the chain reaction remains steady and safe.
Example:
When a uranium-235 nucleus undergoes fission, about 200 MeV of energy is released. If \(1 \, \text{mol}\) of uranium-235 undergoes fission, estimate the total energy released (Avogadro’s number = \(6.02 \times 10^{23}\)).
▶️ Answer/Explanation
Energy per nucleus = \(200 \, \text{MeV} = 200 \times 1.6 \times 10^{-13} \, \text{J} = 3.2 \times 10^{-11} \, \text{J}\).
Number of nuclei in 1 mol = \(6.02 \times 10^{23}\).
Total energy = \( (3.2 \times 10^{-11}) \times (6.02 \times 10^{23}) \approx 1.9 \times 10^{13} \, \text{J} \).
Final Answer: \(\boxed{1.9 \times 10^{13} \, \text{J}}\)
Nuclear Fusion
Occurs when two light nuclei combine to form a heavier nucleus.
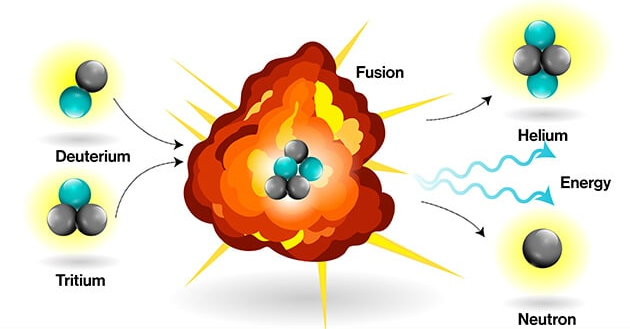
- Releases much more energy per reaction than fission because the binding energy of the product nucleus is very high.
- Powers the Sun and stars.
- Extremely difficult to achieve on Earth due to the very high temperatures and pressures required.
Example reaction: \( ^{2}\text{H} + ^{3}\text{H} \;\;\rightarrow \;\; ^{4}\text{He} + ^{1}\text{n} + \text{Energy} \)
Applications: Hydrogen bombs, potential clean energy source (future reactors like ITER).
Advantages: No long-lived radioactive waste; abundant fuel (hydrogen isotopes); cleaner than fission.
Disadvantages: Requires extremely high temperature and pressure; currently not commercially viable.
Example:
Explain why nuclear fusion is the main energy source of the Sun, and why fusion requires such extreme conditions.
▶️ Answer/Explanation
Fusion combines hydrogen nuclei into helium, releasing energy due to mass-to-energy conversion (\(E = mc^2\)).
Very high temperatures (millions of °C) are needed so nuclei have enough kinetic energy to overcome electrostatic repulsion.
The Sun’s immense gravity provides the pressure and heat necessary for fusion.
Example:
A deuterium-tritium fusion reaction releases about \(17.6 \, \text{MeV}\) per reaction. Estimate the energy released if \(1 \times 10^{20}\) such reactions occur.
▶️ Answer/Explanation
Energy per reaction = \(17.6 \times 1.6 \times 10^{-13} = 2.82 \times 10^{-12} \, \text{J}\).
For \(1 \times 10^{20}\) reactions: \( (2.82 \times 10^{-12}) \times (1 \times 10^{20}) \).
= \(2.82 \times 10^{8} \, \text{J}\).
Final Answer: \(\boxed{2.82 \times 10^{8} \, \text{J}}\)
Nuclear Reactors
Nuclear reactors are machines that control fission reactions to safely release energy for electricity generation.

- Fuel rods (uranium or plutonium) undergo controlled fission reactions.
- Control rods (boron/cadmium) absorb excess neutrons to regulate chain reactions.
- Moderator (water or graphite) slows down neutrons to improve efficiency.
- Coolant transfers heat to water, producing steam to drive turbines.
- Shielding (thick concrete/lead) protects workers and the environment from radiation.
Advantages: Reliable electricity supply; no greenhouse gas emissions during operation.
Disadvantages: Radioactive waste disposal; risk of accidents; high construction/decommissioning costs.
Example:
In a nuclear reactor, why are control rods and moderators necessary for safe and efficient operation?
▶️ Answer/Explanation
Control rods absorb excess neutrons, preventing runaway reactions.
Moderators slow neutrons, making them more effective in causing further fission of uranium-235.
Together, they keep the reaction stable, efficient, and safe.
Example:
Describe the sequence of energy transformations inside a nuclear power plant, starting from nuclear fission up to the production of electricity.
▶️ Answer/Explanation
Step 1: Fission releases nuclear energy as heat.
Step 2: Heat converts water into steam.
Step 3: Steam drives turbines (mechanical energy).
Step 4: Turbines power generators, producing electrical energy.
Final Conversion: Nuclear → Thermal → Kinetic → Electrical.
